
KYNTHEUM 210 mg Injectable Solution in Pre-filled Syringe

How to use KYNTHEUM 210 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Kyntheum210mg solution for injection in pre-filled syringe
brodalumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Kyntheum and what is it used for
- What you need to know before you use Kyntheum
- How to use Kyntheum
- Possible side effects
- Storing Kyntheum
- Contents of the pack and other information
1. What is Kyntheum and what is it used for
Kyntheum contains the active substance brodalumab. Brodalumab is a monoclonal antibody, a distinct type of protein that recognizes and binds to certain proteins in the body.
Brodalumab belongs to a group of medicines known as interleukin inhibitors (IL). This medicine blocks the activity of IL-17 proteins, which are present in high levels in diseases such as psoriasis.
Kyntheum is used to treat a skin disease called "plaque psoriasis", which causes skin inflammation and the formation of scaly plaques. Kyntheum is indicated in adults with moderate to severe plaque psoriasis with extensive body surface area involvement.
Using Kyntheum will help improve skin clearance and reduce the signs and symptoms of psoriasis, such as itching, redness, scaling, burning, stinging, cracking, flaking, and pain.
2. What you need to know before you use Kyntheum
Do not use Kyntheum
- if you are allergic to brodalumab or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, consult your doctor before using Kyntheum.
- if you have active Crohn's disease.
- if you have an infection that your doctor considers important (e.g., active tuberculosis).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Kyntheum:
- if you have an inflammatory disease affecting the intestine called Crohn's disease.
- if you have inflammation of the large intestine called ulcerative colitis.
- if you have ever had or have suicidal thoughts, depression, anxiety, or emotional instability.
- if you have any current or recurring infections.
- if you have any chronic infection.
- if you have tuberculosis (TB), have tested positive for TB, or have been in close contact with someone with TB. You may need to be treated with a TB medicine before starting treatment with Kyntheum.
- if you have recently received or are scheduled to receive any vaccine; certain types of vaccines (live vaccines) should not be given while being treated with Kyntheum.
- if you used Kyntheum during the last three months of pregnancy; in this case, you should consult your doctor before vaccinating your baby.
- if you are receiving any other treatment for psoriasis, such as another immunosuppressant or phototherapy with ultraviolet (UV) light.
After starting treatment with Kyntheum, inform your doctor, pharmacist, or nurse immediately:
- if your doctor has told you that you have developed Crohn's disease.
- if you feel depressed or nervous, have suicidal thoughts, or experience unusual emotional changes.
- if you have any infection or experience any signs of infection listed in section 4, "Possible side effects".
- if you have been told that you have tuberculosis.
Inflammatory bowel disease (Crohn's disease or ulcerative colitis)
Stop taking Kyntheum and inform your doctor or seek medical attention immediately if you notice abdominal pain and cramps, diarrhea, weight loss, or blood in your stool (any sign of intestinal problems).
Monitor for allergic reactions
Kyntheum may potentially cause serious side effects, including allergic reactions. You should monitor for these signs and symptoms while using Kyntheum.
Interrupt treatment with Kyntheum and inform your doctor or seek medical attention immediately if you notice any signs of a possible allergic reaction. These signs are listed in section 4 "Serious side effects".
Children and adolescents
Kyntheum is not recommended for children and adolescents (under 18 years), as it has not been studied in this age group.
Other medicines and Kyntheum
Inform your doctor or pharmacist:
- if you are taking, have recently taken, or might take any other medicine.
- if you have recently received or are scheduled to receive any vaccine; consult "Warnings and precautions" in section 2, "What you need to know before you use Kyntheum".
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Kyntheum has not been studied in pregnant women, and it is not known whether this medicine could harm the fetus. Therefore, it is recommended to avoid using Kyntheum during pregnancy. If you are a woman of childbearing age, you are advised to avoid becoming pregnant and should use adequate contraception while using Kyntheum and for at least 12 weeks after the last dose of Kyntheum.
It is not known whether brodalumab passes into breast milk. If you are breastfeeding or plan to breastfeed, consult your doctor. Your doctor will help you decide whether to stop breastfeeding or stop using Kyntheum, weighing the benefits of breastfeeding for the baby and the benefits of Kyntheum for you.
Driving and using machines
Kyntheum is unlikely to affect your ability to drive or use machines.
3. How to use Kyntheum
Kyntheum should only be prescribed by a doctor with experience in the diagnosis and treatment of psoriasis.
Follow the instructions for administration of this medicine exactly as your doctor, pharmacist, or nurse has told you. If you are unsure, consult your doctor, pharmacist, or nurse again.
How much Kyntheum to use
- Your doctor will decide the dose of Kyntheum you need and the duration of treatment. The recommended dose is 210 mg (one injection).
- After the first dose, you will need to have an injection every week in week 1 (one week after the first dose) and in week 2 (two weeks after the first dose). After that, you will need to have an injection every two weeks.
- Kyntheum is a long-term treatment medicine. Your doctor will periodically check your disease to ensure the treatment has the desired effect. Inform your doctor if you think the signs and symptoms of psoriasis do not improve after using Kyntheum.
How to use Kyntheum
Kyntheum is given by injection under the skin (subcutaneous injection).
Instructions for self-administration
Read the detailed "Instructions for use" provided with this medicine to learn how to store, prepare, and administer the injections correctly at home.
- If your doctor decides that you or a caregiver can administer the injections at home, you will be trained on how to prepare and inject Kyntheum correctly. Do not attempt to inject Kyntheum until your doctor or nurse has shown you or your caregiver how to administer the Kyntheum injections.
- Do not shake the pre-filled syringe before use.
- You or your caregiver can inject Kyntheum into the top of your thighs (thighs) or abdomen (stomach). The caregiver can also give you the injection in the upper outer arm.
- Do not inject the medicine into an area of sensitive, irritated, red, hardened, or psoriasis-affected skin.
If you use more Kyntheum than you should
Inform your doctor if you use more medicine than prescribed or if you have administered the dose earlier than scheduled.
If you forget to use Kyntheum
If you miss a dose of Kyntheum, inject the next dose as soon as possible after the missed dose. Then, ask your doctor when to inject the next dose. Do not administer a double dose to make up for the missed doses.
If you stop using Kyntheum
Do not stop using Kyntheum without talking to your doctor first. If you stop treatment, the symptoms of psoriasis may return.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Stop treatment with Kyntheum and inform your doctor or seek medical attention immediately if you notice any of the following side effects. Your doctor will decide whether you should and when you can restart treatment.
Severe allergic reaction(may affect up to 1 in 1,000 people); symptoms may include:
- difficulty breathing or swallowing
- low blood pressure, which can cause dizziness or fainting
- swelling of the face, lips, tongue, or throat
- intense itching of the skin with rash or blisters.
Serious infections(may affect up to 1 in 100 people); symptoms may include:
- fever, flu-like symptoms, night sweats
- feeling tired or having trouble breathing, persistent cough
- hot, red, painful, or blistering skin
Other side effects
Common (may affect up to 1 in 10 people)
- diarrhea
- nausea
- redness, pain, itching, irritation, or bleeding at the injection site
- fatigue
- sore throat or mouth
- skin infections (e.g., fungal infections, including athlete's foot or jock itch)
- flu (influenza)
- headache
- joint pain
- muscle pain
Uncommon (may affect up to 1 in 100 people)
- Candida (fungal) infection in the mouth, throat, or genitals
- eye discharge with itching, redness, and swelling (conjunctivitis)
- low white blood cell count
Most of these side effects are mild to moderate. If any of these side effects gets serious, inform your doctor, pharmacist, or nurse.
Abdominal pain and cramps, diarrhea, weight loss, or blood in the stool (signs of intestinal problems) have also been reported with IL-17 inhibitors like Kyntheum.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Kyntheum
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging and on the label of the pre-filled syringe after EXP. The expiry date is the last day of the month stated.
Store the pre-filled syringe in the outer packaging to protect it from light.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Kyntheum can be stored at room temperature up to 25°C in the outer packaging for 14 days. Discard Kyntheum if not used within 14 days of storage at room temperature.
Do not use this medicine if you notice that the solution is cloudy, has lost color, or contains particles, flakes, or precipitates.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Kyntheum Composition
- The active ingredient is brodalumab. Each pre-filled syringe contains 210 mg of brodalumab in 1.5 ml of solution.
- The other components are proline, glutamate, polysorbate 20, and water for injectable preparations.
Product Appearance and Container Contents
Kyntheum is a clear or slightly opalescent, colorless or slightly yellowish injectable solution that does not contain particles.
Kyntheum is available in single-unit containers of 2 pre-filled syringes and in multiple-unit containers that contain 3 boxes, each with 2 pre-filled syringes. Not all pack sizes may be marketed.
Marketing Authorization Holder
LEO Pharma A/S
Industriparken 55
DK-2750 Ballerup
Denmark
Manufacturer
Laboratoires LEO
39 route de Chartres
28500 Vernouillet
France
LEO Pharma A/S
Industriparken 55
DK-2750 Ballerup
Denmark
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien LEO Pharma N.V./S.A Tel: +32 3 740 7868 | Lithuania LEO Pharma A/S Tel: +45 44 94 58 88 |
Bulgaria Borola Ltd Tel: +359 2 9156 136 | Luxembourg/Luxemburg LEO Pharma N.V./S.A Tel: +32 3 740 7868 |
Czech Republic LEO Pharma s.r.o. Tel: +420 225 992 272 | Hungary LEO Pharma Tel: +36 1 888 0525 |
Denmark LEO Pharma AB Tel: +45 70 22 49 11 | Malta E.J. Busuttil Ltd Tel: +356 2144 7184 |
Germany LEO Pharma GmbH Tel: +49 6102 2010 | Netherlands LEO Pharma B.V. Tel: +31 205104141 |
Estonia LEO Pharma A/S Tel: +45 44 94 58 88 | Norway LEO Pharma AS Tel: +47 22514900 |
Greece LEO Pharmaceutical Hellas S.A. Tel: +30 210 68 34322 | Austria LEO Pharma GmbH Tel: +43 1 503 6979 |
Spain Laboratorios LEO Pharma, S.A. Tel: +34 93 221 3366 | Poland LEO Pharma Sp. z o.o. Tel: +48 22 244 18 40 |
France LEO Pharma Tel: +33 1 3014 4000 | Portugal LEO Farmacêuticos Lda. Tel: +351 21 711 0760 |
Croatia Remedia d.o.o Tel: +385 1 3778 770 | Romania LEO Pharma Romania Tel: +40 213121963 |
Ireland LEO Laboratories Ltd Tel: +353 (0) 1 490 8924 | Slovenia PHARMAGAN d.o.o. Tel: +386 4 2366 700 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia LEO Pharma s.r.o. Tel: +421 2 5939 6236 |
Italy LEO Pharma S.p.A Tel: +39 06 52625500 | Finland LEO Pharma Oy Tel: +358 20 721 8440 |
Cyprus The Star Medicines Importers Co. Ltd. Tel: +357 2537 1056 | Sweden LEO Pharma AB Tel: +46 40 3522 00 |
Latvia LEO Pharma A/S Tel: +45 44 94 58 88 | United Kingdom(Northern Ireland) LEO Laboratories Ltd Tel: +44 (0) 1844 347333 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for Use:
Kyntheum (brodalumab)
Injectable Solution in Pre-filled Syringe
Subcutaneous Administration
Kyntheum is presented in a single-use pre-filled syringe. Each syringe contains a dose of Kyntheum of 210 mg. Your doctor, pharmacist, or nurse will tell you how often to inject the medication. Each Kyntheum pre-filled syringe can only be used once.
If your doctor decides that you or a caregiver can administer the injections at home, you will be trained on how to prepare and inject Kyntheum correctly. Do not attempt to inject the medication until your doctor has instructed you on how to administer the injections correctly.
Read all the instructions before using the Kyntheum pre-filled syringe.Call your doctor, pharmacist, or nurse if you or your caregiver have any questions about how to inject Kyntheum correctly.
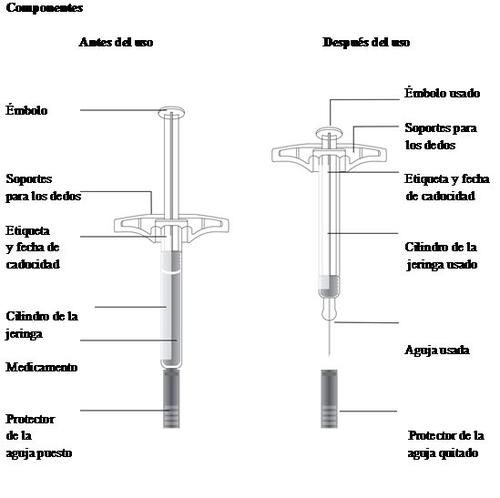
Important:The needle is inside
Important
Before using a Kyntheum pre-filled syringe,read the following instructions:
Storage of Kyntheum Pre-filled Syringes
- Keep out of sight and reach of children.
- Store in the original packaging to protect from light or physical damage.
- Store in the refrigerator (between 2°C and 8°C).
- If necessary, Kyntheum pre-filled syringes can be stored at room temperature (up to 25°C) for a maximum of 14 days. Discard Kyntheum syringes that have been stored at room temperature for more than 14 days.
- Do notfreeze.
Using Kyntheum Pre-filled Syringes
- Do notuse the syringe after the expiration date on the label.
- Do notshake the syringe.
- Do notremove the needle protector until you are ready to administer the injection.
- Do notuse the Kyntheum pre-filled syringe if it has been dropped onto a hard surface. This is in case the syringe is broken.
Step 1: Preparation
- Remove the Kyntheum pre-filled syringe from the box
Hold the syringe by the cylinder to remove it from the tray.
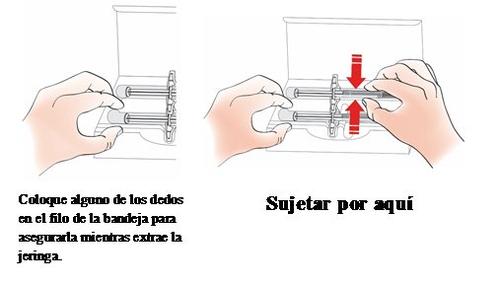
If any syringes are left unused, store the box in the refrigerator.
For safety reasons:
- Do nothold the syringe by the plunger.
- Do nothold the syringe by the needle protector.
- Do notremove the needle protector until you are ready to administer the injection.
- Do notremove the finger grips. They are part of the syringe.
Let the syringe sit at room temperature for at least 30minutes before injecting the medication.
- Do notput the syringe back in the refrigerator if it has already reached room temperature.
- Do nottry to warm the syringe using a heat source, such as hot water or a microwave.
- Do notexpose the syringe to direct sunlight.
- Do notshake the syringe.
Important:Always hold the pre-filled syringe by the cylinder.
- Examine the Kyntheum pre-filled syringe
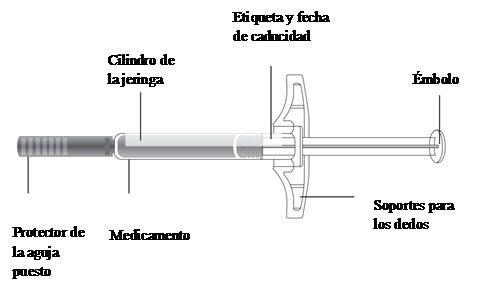
Always hold the syringe by the cylinder.
Make sure the medication in the syringe is clear or slightly opalescent and colorless or slightly yellowish.
- Do notuse the syringe if:
- the medication is cloudy, has lost color, or contains flakes or particles.
- it appears to be broken or cracked in any area.
- Gather all necessary materials
Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place:
- the new syringe.
- alcohol swabs.
- cotton balls or gauze pads.
- band-aids.
- a puncture-resistant container for disposing of needles (the color and appearance of this container may vary depending on national requirements).

- Prepare and clean the injection site
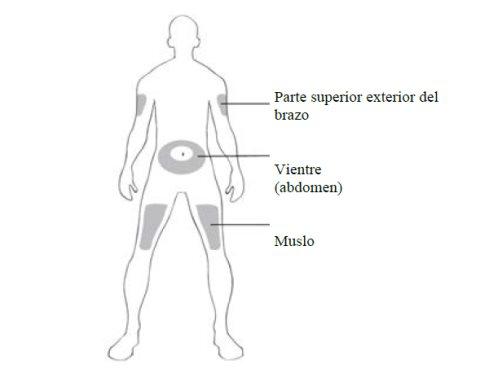
You or your caregiver can administer the injection in:
- the thigh
- the stomach (abdomen), except for the area around the navel (diameter of 5 cm).
Only the caregiver can administer the injection in:
- the upper outer arm.
- Do notinject the medication into areas where the skin is sensitive, irritated, red, or hardened.
- Avoid injecting the medication into areas with scars or stretch marks.
- Avoid injecting the medication directly into areas of thick, red, scaly, or bumpy skin and lesions.
- Clean the area where you will inject the medication with an alcohol swab. Let the skin dry.
- Do nottouch this area again before injecting the medication.
- If you want to use the same injection site, make sure you do not use exactly the same spot you selected for the previous injection.
Step 2: Preparation for Injection
- When you are ready to inject the medication, remove the needle protector with a straight motion away from your body
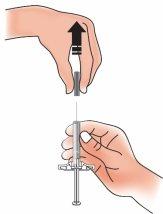
Discard the needle protector in the puncture-resistant container.
- Do notbend or twist the needle protector.
- Do notput the needle protector back on.
You may see small air bubbles in the syringe or a drop of liquid on the tip of the needle. Both are normal and do not need to be removed.
- Pinch the skin to create a firm surface
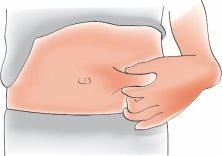
Hold the skin firmly with your thumb and other fingers and create an area about 5 cm wide.
Important:Continue to hold the skin until you have injected the medication.
Step 3: Injection
- Hold the skin. Once you have removed the needle protector, insert the syringe into the skin at an angle between 45 and 90 degrees
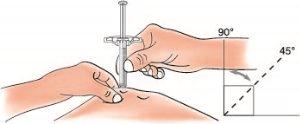
Do notplace your finger on the plunger while inserting the needle.
- Apply slow but constant pressure on the plunger until it stops moving
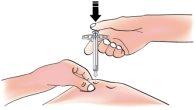
- When you are finished, release your thumb. Then, carefully remove the syringe from the skin
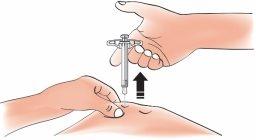
Important:If, when removing the syringe, you see that there is still medication in the syringe cylinder, the full dose has not been administered. Call your doctor, pharmacist, or nurse immediately.
Step 4: Completion
- Discard the used syringe
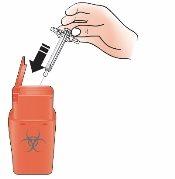
- Discard the used Kyntheum pre-filled syringe in a puncture-resistant container immediately after use.
- Do notreuse the syringe.
- Do notrecycle the syringe or the puncture-resistant container, or throw them in the trash.
Important:Keep the puncture-resistant container out of sight and reach of children.
- Check the injection site
If there is bleeding, press the injection site with a cotton ball or gauze pad. Do notrub the injection site. Cover it with a band-aid if necessary.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KYNTHEUM 210 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 90 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for KYNTHEUM 210 mg Injectable Solution in Pre-filled Syringe
Discuss questions about KYNTHEUM 210 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






