JIVI 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use JIVI 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for theuser
Jivi 250 UI powder and solvent for solutionfor injection
Jivi 500 UI powder and solvent for solutionfor injection
Jivi 1000 UI powder and solvent for solutionfor injection
Jivi 2000 UI powder and solvent for solutionfor injection
Jivi 3000 UI powder and solvent for solutionfor injection
human recombinant coagulation factor VIII pegylated with B domain suppressed (damoctocog alfa pegol)
Read the entire package leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Jivi and what is it used for
- What you need to know before using Jivi
- How to use Jivi
- Possible side effects
- Storage of Jivi
- Package contents and additional information
1. What is Jivi and what is it used for
Jivi contains the active substance damoctocog alfa pegol. It is produced using recombinant technology without the addition of any human or animal-derived components in the manufacturing process. Factor VIII is a protein that occurs naturally in the blood and helps it to clot. The damoctocog alfa pegol protein has been modified (pegylated) to prolong its action in the body.
Jivi is used to treat and prevent bleedingin adults and adolescents aged 12 years or older with haemophilia A (hereditary factor VIII deficiency) who have been previously treated. It is not for use in children under 12 years of age.
2. What you need to know before using Jivi
Do not use Jivi if you are:
- allergic to damoctocog alfa pegol or any of the other components of this medicine (listed in section 6).
- allergic to mouse or hamster proteins.
Warnings and precautions
Consult your doctor or pharmacist if:
? you experience chest tightness, a drop in blood pressure (often manifested by a feeling of dizziness when standing up quickly), urticaria with itching, wheezing (whistling when breathing), general malaise or fainting. These may be signs of a severe allergic reactionto this medicine. Stopthe injection of the medicineimmediately and seek medical attention if this occurs.
- you have a bleed that does not respond to your usual dose of this medicine. Talk to your doctor immediately if this happens. You may have developed antibodies against factor VIII (inhibitors) or antibodies against polyethylene glycol (PEG). These antibodies reduce the effectiveness of Jivi in preventing and controlling bleeding. Your doctor may perform tests to confirm this possibility and ensure that your dose of Jivi generates adequate levels of factor VIII. If necessary, your doctor may switch you back to the previous factor VIII treatment.
- you have previously developed factor VIII inhibitors with a different product.
- you have a history of heart disease or are at risk of heart disease.
- you need to use a central venous access device for this medicine. You may be at risk of complications related to the device in the area where the catheter is inserted, including:
- local infections
- bacteria in the blood
- formation of a blood clot in the blood vessel
Children
Jivi should not be used in children under 12 years of age.
Other medicines and Jivi
It is not known whether Jivi affects or is affected by other medicines. Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines
Jivi has no influence on the ability to drive and use machines.
Jivi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; i.e., it is essentially "sodium-free".
3. How to use Jivi
Treatment with Jivi will be started by a doctor experienced in the care of patients with haemophilia A. After receiving the relevant training, patients or caregivers may be able to administer Jivi at home. Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again. The dose of factor VIII is measured in International Units (IU).
Treatment of bleeding
To treat bleeding, your doctor will calculate and adjust your dose and how often it should be administered, depending on factors such as:
- your weight
- the severity of your haemophilia A
- the location and severity of the bleeding
- whether you have factor VIII inhibitors and how high these levels are
- the required level of factor VIII
Prevention of bleeding
To prevent bleeding, your doctor will select a suitable dose and frequency based on your needs:
- 45-60 IU per kg body weight every 5 days or
- 60 IU per kg body weight every 7 days or
- 30-40 IU per kg body weight twice a week.
Laboratory tests
Regular laboratory tests help ensure that you always have adequate levels of factor VIII. In the case of major surgical interventions, your blood coagulation should be closely monitored.
Duration of treatment
Generally, treatment for haemophilia with Jivi will be necessary for life.
How Jivi is administered
Jivi is injected into a vein over 2-5 minutes, depending on the total volume and your level of comfort. The maximum rate is 2.5 ml per minute. Jivi should be used within 3 hours after reconstitution.
How to prepare Jivi for injection
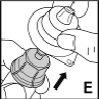
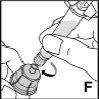
Only use the components (vial adapter, pre-filled syringe with solvent, and venipuncture equipment) included in the package for this medicine. Consult your doctor if it is not possible to use these components. If any of the package components are open or damaged, do not use that component.
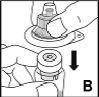
The reconstituted medicine must be filtered using the vial adapterbefore injection to remove any particles that may be present in the solution.
This medicine must notbe mixed with other injections. Do not use solutions that are cloudy or contain visible particles. Follow the instructions for useprovided by your doctor and those at the end of this package leaflet.
If you use more Jivi than you should
Inform your doctor if this happens. No symptoms of overdose have been reported.
If you forget to use Jivi
Inject the next dose immediately and continue at regular intervals, following your doctor's instructions.
Do not use a double dose to make up for forgotten doses.
If you stop treatment with Jivi
Do not stop using this medicine without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most seriousside effects are allergic reactionsor a severe allergic reaction. Stop the injection of Jivi immediately and talk to your doctor right away if this happens.The following symptoms could be an early sign of these reactions:
- chest tightness or general feeling of discomfort
- burning and itching at the injection site
- urticaria, flushing
- drop in blood pressure that can cause dizziness when standing up
- nausea
In patients who have received previous treatment with factor VIII (more than 150 days of treatment), inhibitor antibodies (see section 2) may form in rare cases (less than 1 in 100 patients). If this happens, your medicine may stop working well, and you may experience persistent bleeding. If this happens, contact your doctor immediately.
The following side effects may occur with this medicine:
Very common(may affect more than 1 in 10 people):
- headache
Common(may affect up to 1 in 10 people):
- stomach pain
- nausea, vomiting
- fever
- allergic reactions (which can occur in the form of hives, generalised urticaria, chest tightness, wheezing, shortness of breath, drop in blood pressure; for initial symptoms, see above)
? local reactions at the injection site, such as bleeding under the skin, severe itching, swelling, burning sensation, transient redness
- dizziness
- difficulty sleeping
- cough
- rash, skin redness
Uncommon(may affect up to 1 in 100 people):
- factor VIII inhibition
- taste disturbance
- flushing
- itching
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this package leaflet. You can also report them directly to the Spanish Medicines Agency's website www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Jivi
Keep this medicine out of the sight and reach of children.
Do notuse this medicine after the expiry date stated on the labels and cartons. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do notfreeze.
Keep the vial and pre-filled syringe in the outer packaging to protect from light.
This medicine can be stored at room temperature (below 25°C) for a maximum of 6 months, if kept in the package. If stored at room temperature, it expires after 6 months or on the expiry date stated, whichever is earlier.
You must write the new expiry date on the outer packaging when you remove the medicine from the refrigerator.
Do notrefrigerate the solution after reconstitution. The reconstituted solution must be used within 3 hours.
Do notuse this medicine if you notice particles or if the solution is cloudy.
This medicine is for single use only. Discard any unused solution.
Medicines should notbe disposed of via wastewater or household waste. Ask your pharmacist or doctor how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container contents and additional information
Jivi composition
- The active substance is human recombinant coagulation factor VIII pegylated with suppressed B domain (damoctocog alfa pegol). Each Jivi vial contains 250 or 500 or 1000 or 2000 or 3000 IU of damoctocog alfa pegol. After reconstitution with the supplied solvent (sterile water for injectable preparations), the prepared solutions have the following concentration:
Dose | Approximate concentration after reconstitution |
250 IU | (100 IU / ml) |
500 IU | (200 IU / ml) |
1,000 IU | (400 IU / ml) |
2,000 IU | (800 IU / ml) |
3,000 IU | (1,200 IU / ml) |
- The other components are sucrose, histidine, glycine, sodium chloride (see section 2 "Jivi contains sodium"), calcium chloride dihydrate, polysorbate 80, glacial acetic acid, and water for injectable preparations.
Appearance of the product and container contents
Jivi is presented as a powder and solvent for solution for injection. The powder is dry and white to slightly yellow. The solvent is a clear liquid. After reconstitution, the solution is clear.
Each Jivi container contains
- a glass vial with powder
- a pre-filled syringe with solvent
- a separate plunger rod
- a vial adapter
- a venipuncture set
Jivi is available in containers of:
- 1 individual container
- 1 multiple container with 30 individual containers
Not all pack sizes may be marketed.
Marketing authorisation holder
Bayer AG
51368 Leverkusen
Germany
Manufacturer
Bayer AG
Kaiser-Wilhelm-Allee
51368 Leverkusen
Germany
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel. +37 05 23 36 868 |
???????? ????? ???????? ???? T??.: +359-(0)2 4247280 | Luxembourg/Luxemburg Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 |
Ceská republika Bayer s.r.o. Tel: +420 266 101 111 | Magyarország Bayer Hungária KFT Tel:+36 14 87-41 00 |
Danmark Bayer A/S Tlf: +45 45 23 50 00 | Malta Alfred Gera and Sons Ltd. Tel: +35 621 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49 (0)214-30 513 48 | Nederland Bayer B.V. Tel: +31-23-799 1000 |
Eesti Bayer OÜ Tel: +372 655 8565 | Norge Bayer AS Tlf: +47 23 13 05 00 |
Ελλ?δα Bayer Ελλ?ς ΑΒΕΕ Τηλ: +30-210-61 87 500 | Österreich Bayer Austria Ges.m.b.H. Tel: +43-(0)1-711 46-0 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48 22 572 35 00 |
France Bayer HealthCare Tél (N° vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351 21 416 42 00 |
Hrvatska Bayer d.o.o. Tel: +385-(0)1-6599 900 | România SC Bayer SRL Tel: +40 21 529 59 00 |
Ireland Bayer Limited Tel: +353 1 216 3300 | Slovenija Bayer d. o. o. Tel: +386 (0)1 58 14 400 |
Ísland Icepharma hf. Sími: +354 540 8 000 | Slovenská republika Bayer spol. s r.o. Tel. +421 2 59 21 31 11 |
Italia Bayer S.p.A. Tel: +39 02 397 8 1 | Suomi/Finland Bayer Oy Puh/Tel: +358- 20 785 21 |
Κ?προς NOVAGEM Limited Τηλ: +357 22 48 38 58 | Sverige Bayer AB Tel: +46 (0) 8 580 223 00 |
Latvija SIA Bayer Tel: +371 67 84 55 63 | United Kingdom Bayer plc Tel: +44-(0)118 206 3 000 |
Date of last revision of this leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
------------------------------------------------------------------------------------------------------------------
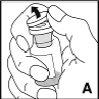
Detailed instructions for reconstitution and administration of Jivi
You will need sterile alcohol-impregnated swabs, sterile swabs, dressings, and a tourniquet. These items are not included in the Jivi container.
| |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
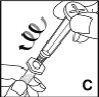
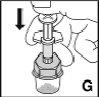
Check that all the contents of the vial have passed into the syringe. Hold the syringe vertically and press the plunger until there is no air in the syringe. |
|
| |
| |
| |
|
|
| |
| |
| |
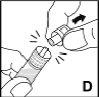
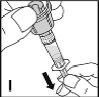
Finally, apply gentle pressure with a swab to the injection site and, if necessary, apply a dressing. | |
| |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to JIVI 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for JIVI 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about JIVI 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions