
IXIARO INJECTABLE SUSPENSION

How to use IXIARO INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
IXIARO injectable suspension
Vaccine against Japanese encephalitis (inactivated, adsorbed)
Read all of this leaflet carefully before you or your child start using this vaccine, because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor.
- This vaccine has been prescribed to you or your child. Do not pass it on to others.
- If you or your child experience any side effects, talk to your doctor, even if you think the side effects are not caused by the vaccine. See section 4.
Contents of the package leaflet:
- What is IXIARO and what is it used for
- What you need to know before you or your child use IXIARO
- How to use IXIARO
- Possible side effects
- Storage of IXIARO
- Contents of the pack and other information
1. What is IXIARO and what is it used for
IXIARO is a vaccine against the Japanese encephalitis virus.
The vaccine causes the body to create its own protection (antibodies) against this disease.
IXIARO is indicated to prevent infection with the Japanese encephalitis virus (JEV). This virus is mainly found in Asia and is transmitted to humans through mosquitoes that have bitten an infected animal (e.g., a pig). Many people infected have mild symptoms or no symptoms. In people who develop a severe form of the disease, JE usually starts like the flu, with fever, chills, fatigue, headache, nausea, and vomiting. In the early stages of the disease, confusion and agitation also appear.
IXIARO should only be administered to adults, adolescents, children, or infants over 2 months of age who are traveling to countries where JE is endemic or who are at risk of contracting it due to their work.
2. What you need to know before you or your child start using IXIARO
DO NOT use IXIARO:
- If you or your child are allergic (hypersensitive) to the active substance or any of the other components of this medicine (listed in section 6).
- If you or your child have experienced an allergic reaction after receiving a previous dose of IXIARO. The symptoms of an allergic reaction may include a skin rash with itching, difficulty breathing, and swelling of the face and tongue.
- If you or your child have a disease with high fever. In this case, your doctor will postpone vaccination.
Warnings and precautions
IXIARO should not be injected into a blood vessel.
Primary vaccination should be completed at least one week before possible exposure to JEV.
Tell your doctor:
- If you or your child have experienced any health problems after administration of any previous vaccine;
- If you or your child have any other known allergy;
- If you or your child have a bleeding disorder (a disease that makes you or your child bleed more than normal) or have a decrease in blood platelets, which increases the risk of bleeding or bruising (thrombocytopenia);
- If your child is under 2 months of age, as IXIARO has not been tested in infants under 2 months;
- If your immune system (you or your child) does not work properly (immunodeficiency) or if you or your child are taking medications that affect your immune system (such as a medication called cortisone or cancer medications).
Your doctor will explain the possible risks and benefits of receiving IXIARO.
Keep in mind the following:
- IXIARO cannot cause the disease it protects against.
- IXIARO will not prevent infections caused by other viruses different from the Japanese encephalitis virus.
- Like any other vaccine, vaccination with IXIARO may not provide protection in all cases.
- You should take necessary precautions to avoid you or your child getting mosquito bites (using suitable clothing, repellents, mosquito nets) even after being vaccinated with IXIARO.
Using IXIARO with other medicines
Studies in humans to evaluate the efficacy and safety of medicines (clinical trials) have shown that IXIARO can be administered at the same time as the hepatitis A vaccine and the rabies vaccine.
Tell your doctor if you or your child are using, have recently used, or may use other medicines, including those obtained without a prescription, or if you or your child have recently received any other vaccine.
Pregnancy, breastfeeding, and fertility
There is not enough data on the use of IXIARO in pregnant or breastfeeding women.
As a precaution, IXIARO should not be used during pregnancy or breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this vaccine.
Driving and using machines
IXIARO has no influence or insignificant influence on the ability to drive and use machines.
IXIARO contains potassium and sodium
This medicine contains potassium, less than 1 mmol (39 mg) per 0.5 ml single dose, i.e., essentially "potassium-free") and less than 1 mmol of sodium (23 mg) per 0.5 ml single dose, i.e., essentially "sodium-free"). This medicine may contain residues of sodium metabisulfite, which are below the detection limit
3. How to use IXIARO
The recommended dose for adults, adolescents, and children over 3 years of age is 2 injections of 0.5 ml each:
- The first injection, on day 0
- The second injection, 28 days after the first (day 28)
Adults aged 18 to ≤ 65 years can also be vaccinated as follows:
- The first injection on day 0
- The second injection 7 days after the first injection (day 7).
Infants and children between 2 months and <3 years of age< p>
The recommended dose for infants and children between 2 months and <3 years of age is 2 injections 0.25 ml each:< p>
- The first injection, on day 0
- The second injection, 28 days after the first (day 28)
To obtain instructions on preparing a 0.25 ml dose, see the end of this leaflet.
Make sure you or your child complete the full vaccination program of 2 injections. The second injection should be administered at least one week before possible exposure to the Japanese encephalitis virus. Otherwise, you or your child may not be fully protected against the disease.
In the case of adults, adolescents, children, and infants over 1 year of age, a booster dose can be administered during the second year (i.e., 12 to 24 months) after the first dose of the recommended primary immunization. In the case of adults, a second booster dose can be administered 10 years after the first booster dose. In the case of elderly persons (>65 years), the first booster dose may be administered earlier. Your doctor will decide on the requirement and timing of booster doses.
Administration
Your doctor or nurse will inject IXIARO into you or your child in the muscle of the arm (deltoid). IXIARO should not be injected into a blood vessel. If you or your child have a bleeding disorder, your doctor may decide to administer the vaccine under the skin (subcutaneously).
If you have any other questions about the use of this product, ask your doctor or pharmacist.
If you miss a dose of IXIARO
If you or your child miss one of the scheduled injections, consult your doctor and schedule another appointment for the second injection. Without the second injection, you or your child will not be fully protected against the disease. There is information indicating that the second injection can be administered up to 11 months after the first.
4. Possible side effects
Like all medicines, this product can cause side effects, although not everybody gets them.
During clinical trials, most of the following side effects have been observed, which usually occur within three days of vaccination, are generally mild, and disappear within a few days.
Very common (affect more than 1 user in 10):
Headache, muscle pain, pain at the injection site, hypersensitivity at the injection site, fatigue
Common (affect between 1 and 10 users in 100):
Nausea, flu-like illness, fever, other reactions at the injection site (e.g., redness, hardness, swelling, itching)
Uncommon (affect between 1 and 10 users in 1000):
Vomiting, skin rash, changes in lymph nodes, migraine (pulsating headache, often accompanied by nausea and vomiting and sensitivity to light), dizziness, vertigo (feeling that everything is spinning), diarrhea, abdominal pain, excessive sweating, itching, chills, feeling of general discomfort, musculoskeletal stiffness, joint pain, weakness, abnormal liver test results (elevated liver enzymes)
Rare (affect between 1 and 10 users in 10,000):
Palpitations, rapid heart rate, difficulty breathing, abnormal sensation in the skin (e.g., pinching), hives, skin redness, pain in the legs or arms, platelet deficiency, nerve inflammation, swelling of the extremities and ankles, taste disturbance, swelling of the eyelids, fainting
Other side effects in children between 2 months and <3 years of age< strong>
In children between 2 months and <3 years of age, the following side effects have been observed more frequently compared to children between 3 and <12 years, adolescents, adults:< p>
Very common:fever (28.9%), diarrhea (11.8%), flu-like illness (11.2%), irritability (11.0%)
Common:loss of appetite, vomiting, rash
Uncommon:cough
Reporting of side effects
If you or your child experience any side effects, talk to your doctor, even if you think the side effects are not caused by the vaccine. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of IXIARO
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the carton after "EXP". The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze. If the vaccine has been frozen, it must not be used.
- Store in the original package to protect from light.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. ADDITIONAL INFORMATION
Composition of IXIARO
One dose (0.5 ml) of IXIARO contains:
6 AU3 of the SA14-14-2 strain (inactivated) of the Japanese encephalitis virus1,2
equivalent to a potency ≤ 460 ng DE50
- produced in Vero cells
- adsorbed on hydrated aluminum hydroxide (approximately 0.25 milligrams Al3+)
- Antigen units
Aluminum hydroxide is added to this vaccine as an adjuvant.
The other components are: sodium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate, water for injections
Appearance and packaging of the product
IXIARO is an injectable suspension (0.5 ml in a glass syringe with or without a needle supplied separately, pack with 1 syringe).
IXIARO is a sterile white or slightly milky suspension that becomes homogeneous when shaken.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Valneva Austria GmbH
Campus Vienna Biocenter 3
A-1030 Vienna
Austria
Email: [email protected]
Manufacturer:
Valneva Scotland Ltd.
Oakbank Park Road,
Livingston EH53 0TG
United Kingdom
Valneva Austria GmbH
Campus Vienna Biocenter 3
A-1030 Vienna
Austria
Date of last revision of this leaflet.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/. On the European Medicines Agency website, this leaflet can be found in all languages of the European Union/European Economic Area.
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The pre-filled syringe is for single use and should not be used in more than one person. The pre-filled syringe is ready to use. If a needle is not supplied, use a sterile needle.
Do not use if the blister pack is damaged or the packaging is damaged.
During the storage period, a slight white deposit may form with a clear and transparent supernatant.
Before administration, shake the syringe well to obtain a homogeneous white suspension. Do not administer if particles or color changes are observed after shaking or if the syringe is physically damaged.
Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
Information about administering a 0.5 ml dose of IXIARO to persons over 3 years of age
In the case of administering a full 0.5 ml dose, follow these steps:
- Shake the syringe until a homogeneous suspension is obtained.
- Remove the cap from the tip of the syringe by gently twisting it. Do not attempt to break or pull the tip, as this could damage the syringe.
- Attach a needle to the pre-filled syringe.
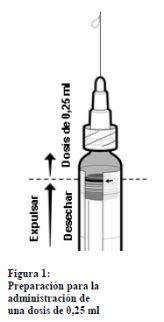
Information about preparing a 0.25 ml dose of IXIARO for use in children under 3 years of age
In the case of administering a 0.25 ml dose to children between 2 months and <3 years of age, follow these steps:< p>
- Shake the syringe until a homogeneous suspension is obtained.
- Remove the cap from the tip of the syringe by gently twisting it. Do not attempt to break or pull the tip, as this could damage the syringe.
- Attach a needle to the pre-filled syringe.
- Hold the syringe in a vertical position.
- Push the plunger stopper to the edge of the red line on the syringe tube, indicated by a red arrow (see Figure 1)* to discard the excess volume
- Attach a new sterile needle before injecting the remaining volume.
*If you push the plunger stopper beyond the red line, the 0.25 ml dose cannot be guaranteed, and a new syringe must be used.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IXIARO INJECTABLE SUSPENSIONDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 3.75 microgramsActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, UnknownActive substance: combinationsManufacturer: Glaxosmithkline BiologicalsPrescription required
Online doctors for IXIARO INJECTABLE SUSPENSION
Discuss questions about IXIARO INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















