
ISOPROTRACE 10 MICROGRAMS KIT FOR RADIOPHARMACEUTICAL PREPARATION
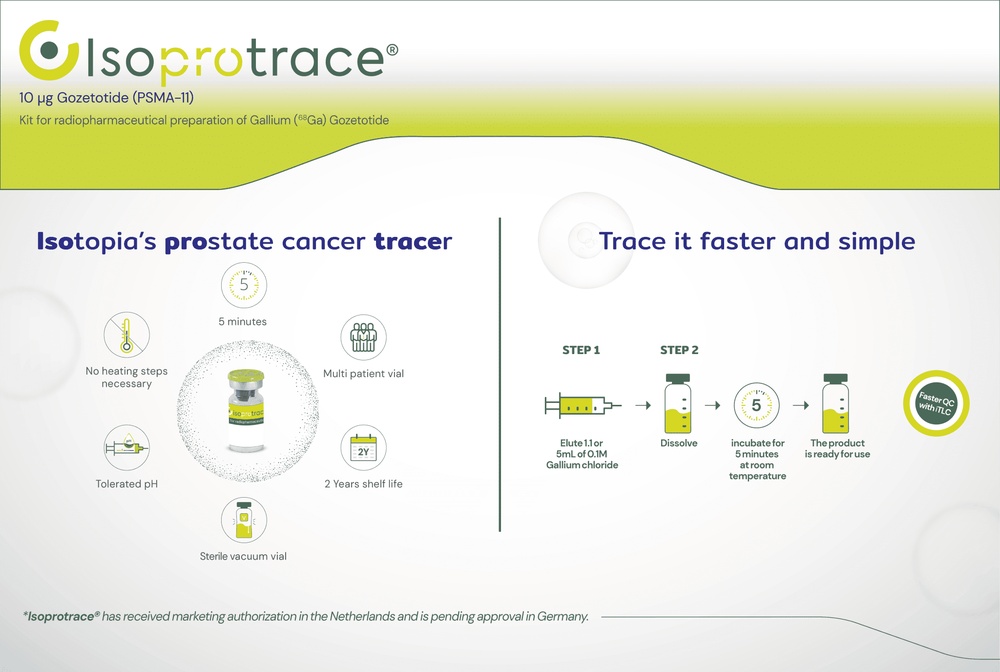

How to use ISOPROTRACE 10 MICROGRAMS KIT FOR RADIOPHARMACEUTICAL PREPARATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Isoprotrace 10micrograms kit for radiopharmaceutical preparation
gozetotida
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your nuclear medicine doctor who will be supervising the procedure.
- If you experience side effects, consult your nuclear medicine doctor, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What Isoprotrace is and what it is used for
- What you need to know before you are given Isoprotrace
- How Isoprotrace is administered
- Possible side effects
- Storage of Isoprotrace
- Contents of the pack and further information
1. What Isoprotrace is and what it is used for
This medicine is a radiopharmaceutical for diagnostic use only.
It contains a substance called gozetotida. Before it can be used, the powder in the vial is mixed with a radioactive substance called gallium (68Ga) chloride to produce gallium (68Ga)-gozetotida (this process is called radiolabelling).
After radiolabelling with gallium (68Ga), Isoprotrace is used in a special type of imaging diagnostic technique called positron emission tomography (PET) to detect certain types of cancer cells that have a protein called prostate-specific membrane antigen (PSMA) in patients:
- with prostate cancer who are at high risk of the disease spreading to other parts of the body and who are suitable for treatment that can cure the cancer
- who have received previous treatment for prostate cancer and in whom it is suspected that the cancer has come back, taking into account the results of other tests (e.g. prostate-specific antigen [PSA]).
The use of Isoprotrace involves receiving a small amount of radioactivity. Your doctor and nuclear medicine doctor have considered that the clinical benefit you will gain from the procedure with the radiopharmaceutical outweighs the risk of radiation.
2. What you need to know before you are given Isoprotrace
You should not be given Isoprotrace
- if you are allergic to gozetotida or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to receive Isoprotrace, talk to your nuclear medicine doctor if you have kidney disease.
Before administration of Isoprotrace
You should drink plenty of water before starting the procedure to urinate frequently during the first few hours after it finishes to ensure that Isoprotrace is eliminated from your body as soon as possible.
Children and adolescents
Isoprotrace is not indicated for use in children and adolescents under 18 years of age. The safety and efficacy of gallium (68Ga)-gozetotida have not been established in this patient population.
Pregnancy and breastfeeding
Isoprotrace is not indicated for use in women. All radiopharmaceuticals, including Isoprotrace, may cause harm to the fetus.
Driving and using machines
It is considered unlikely that Isoprotrace will affect your ability to drive and use machines.
Isoprotrace contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial; this is essentially "sodium-free".
3. How Isoprotrace is administered
There are strict rules about the use, handling, and disposal of radiopharmaceuticals. Isoprotrace will only be used in specially controlled areas. This product will only be handled and administered by trained and qualified personnel who are qualified to use it safely. They will take special care in the safe use of this product and will inform you of their actions.
Your nuclear medicine doctor will decide the amount of Isoprotrace to be used in your case. This will be the minimum amount necessary to obtain the desired information.
The recommended dose for administration to an adult is 111 to 259 MBq (megabecquerels, the unit used to express radioactivity).
Administration of Isoprotrace and performance of the procedure
After radiolabelling, Isoprotrace is administered by slow intravenous injection. A single injection is sufficient to perform the test that your doctor needs.
Duration of the procedure
Your nuclear medicine doctor will inform you about the usual duration of the procedure.
After administration of Isoprotrace, you should
- continue to drink plenty of water to stay hydrated and urinate as frequently as possible to eliminate the medicine from your body
- avoid direct contact with small children and pregnant women during the first 6 hours after the injection.
Your nuclear medicine doctor will inform you if you need to take any special precautions after receiving this medicine. Consult your nuclear medicine doctor if you have any doubts.
If you have been given more Isoprotrace than you should
It is unlikely that an overdose will occur, as you will only receive a single dose of Isoprotrace that is precisely controlled by the nuclear medicine doctor supervising the procedure. However, if an overdose occurs, you will receive the appropriate treatment. Drinking and urinating frequently will help to eliminate the radioactivity from your body more quickly.
If you have any further questions about the use of Isoprotrace, ask your nuclear medicine doctor who is supervising the procedure.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur:
Uncommon (may affect up to 1 in 100 people):
- headache
- dizziness
- tingling and numbness of the skin
- feeling of drowsiness during the day or difficulty sleeping at night
- nausea
- diarrhea
- difficulty swallowing
- rash
- fatigue
- burning, itching, and pain at the injection site
Administration of this radiopharmaceutical involves receiving a small amount of ionizing radiation with a very low risk of developing cancer and genetic disorders.
Reporting of side effects
If you experience any side effects, consult your nuclear medicine doctor, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Isoprotrace
You will not need to store this medicine. This medicine is stored under the responsibility of the specialist in suitable facilities. The storage of radiopharmaceuticals will be carried out in accordance with national regulations on radioactive materials.
The following information is intended only for the specialist:
Do not use this medicine after the expiry date stated on the packaging and label after "EXP". The expiry date is the last day of the month indicated.
Store in a refrigerator (2°C - 8°C).
After reconstitution and radiolabelling: store in a vertical position below 25°C and use within 4 hours.
From a microbiological point of view, unless the method of opening, radiolabelling, or dilution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
6. Contents of the pack and further information
Composition of Isoprotrace
- The active substance is gozetotida. Each vial contains 10 micrograms of gozetotida.
- The other excipients are: hydrolyzed gelatin, sodium acetate anhydrous, and sodium chloride. See section 2 "Isoprotrace contains sodium".
After radiolabelling, the resulting solution also contains, as an excipient, hydrochloric acid.
Appearance of Isoprotrace and contents of the pack
The pack contains 5 glass multidose vials with a volume of 10 ml in a cardboard box. Each vial contains a white or off-white powder.
The radioactive substance is not part of the kit and will be added during the preparation steps before administration.
Marketing authorisation holder
Billev Pharma ApS
Slotsmarken 10
2970 Hørsholm
Denmark
Manufacturer
Cilatus Manufacturing Services Limited
Pembroke House
28-32 Pembroke Street Upper
Dublin 2, D02 EK84
Ireland
You can obtain further information on this medicine from thelocal representativeof the marketing authorisation holder:
NUCLIBER, S.A.
Phone: +34 915062940
Date of last revision of this leaflet: 02/2025.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The full summary of product characteristics of Isoprotrace is included as a separate document in the product packaging, in order to provide healthcare professionals with additional scientific and practical information on the administration and use of this radiopharmaceutical.
Please consult the summary of product characteristics.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ISOPROTRACE 10 MICROGRAMS KIT FOR RADIOPHARMACEUTICAL PREPARATIONDosage form: RADIOPHARMACEUTICAL, 25 microgramsActive substance: gallium (68Ga) gozetotideManufacturer: Telix InnovationsPrescription requiredDosage form: RADIOPHARMACEUTICAL, 25 microgramsActive substance: gallium (68Ga) gozetotideManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 74 MBq iobenguane (123I)Active substance: iobenguane (123I)Manufacturer: Ge Healthcare Bio-Sciences, S.A.U.Prescription required
Online doctors for ISOPROTRACE 10 MICROGRAMS KIT FOR RADIOPHARMACEUTICAL PREPARATION
Discuss questions about ISOPROTRACE 10 MICROGRAMS KIT FOR RADIOPHARMACEUTICAL PREPARATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












