
INTIZOL VAGINAL 1.000 mg VAGINAL SUPPOSITORIES

How to use INTIZOL VAGINAL 1.000 mg VAGINAL SUPPOSITORIES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Intizol Vaginal 1,000 mg Vaginal Tablets
metronidazole
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Intizol Vaginal and what is it used for
- What you need to know before you use Intizol Vaginal
- How to use Intizol Vaginal
- Possible side effects
- Storage of Intizol Vaginal
- Contents of the pack and other information
1. What is Intizol Vaginal and what is it used for
Intizol Vaginal is a medicine that belongs to the group of 5-nitroimidazole antibiotics.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or the common cold. It is essential that you follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not dispose of medicines via wastewater or household waste. |
It is used to treat bacterial vaginosis (an alteration in the bacteria of the vagina that may be accompanied by vaginal discharge with a bad odor) in adult women.
2. What you need to know before you use Intizol Vaginal
Do not use Intizol Vaginal
- If you are allergic to metronidazole, other imidazoles, soy, peanuts, or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Intizol Vaginal if:
- You have severe liver disorders, blood formation disorders, brain or spinal cord diseases, or nervous system disorders. In these cases, your doctor will carefully evaluate whether treatment with this medicine is suitable.
- You have Cockayne syndrome. Your doctor will regularly monitor your liver function during and after treatment with metronidazole. There have been reports of severe liver toxicity/acute liver failure, including cases with a fatal outcome, in patients with Cockayne syndrome who received metronidazole-containing medications.
Tell your doctor immediately and stop using metronidazole if you experience:
- Stomach pain, loss of appetite, nausea, vomiting, fever, malaise, fatigue, jaundice, dark urine, light-colored stools, or itching.
- You use latex condoms or diaphragms, as concurrent use with this medicine may reduce the effectiveness of latex contraceptives.
- You are going to have a blood test. This medicine may alter the results of some blood tests.
Tell your doctor immediately and stop taking metronidazole if:
- You experience difficulty breathing, dizziness, and vomiting that may occur in very rare cases of severe allergy (anaphylactic shock). You should seek urgent medical attention and immediately discontinue treatment with this medicine.
- You have severe and prolonged diarrhea during treatment or in the first few weeks after completing it. This can be a symptom of a severe intestinal disease (pseudomembranous colitis) that requires medical attention as soon as possible.
This medicine may darken the color of your urine.
Children and adolescents
This medicine must not be used in girls and adolescents under 18 years of age.
Other medicines and Intizol Vaginal
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
When using this medicine, approximately 20% of the active ingredient (metronidazole) enters the bloodstream. For this reason, the interactions that occur with the oral administration of metronidazole-containing medications are listed below.
Other medicines that influence the effect of Intizol Vaginal
Concomitant administration of metronidazole with disulfiram (used in the treatment of alcoholism) may cause confusion or certain mental disorders (psychosis).
Metronidazole may be less effective when taken with phenobarbital (a barbiturate used for sleep disorders, seizures, and anesthesia) or phenytoin (used to treat seizures and some types of arrhythmias).
In isolated cases, cimetidine (used to treat digestive disorders such as acidity and ulcers) may increase the effect and toxicity of metronidazole.
Effect of Intizol Vaginal on other medicines
Metronidazole may enhance the effect of certain medicines that prevent blood clotting (anticoagulants such as warfarin or acenocoumarol), so it may be necessary to adjust the dose of the anticoagulant.
Concomitant administration of metronidazole and cyclosporin (used in transplants and immune system disorders) may increase blood levels of cyclosporin, increasing its effect and toxicity. Therefore, cyclosporin blood levels and kidney function should be closely monitored.
Metronidazole reduces the elimination of 5-fluorouracil (a chemotherapeutic agent for tumor treatment), which may increase its toxicity when administered simultaneously.
Metronidazole may significantly increase the toxicity of busulfan (a chemotherapeutic agent) when administered concomitantly. Concomitant administration is not recommended due to the risk of severe toxicity and death.
Concomitant administration of metronidazole and lithium (used as a mood stabilizer in bipolar disorder and major depressive disorder) may increase lithium blood levels, posing a risk of lithium intoxication. Therefore, lithium blood levels and kidney function should be closely monitored.
Concomitant administration of metronidazole with tacrolimus (a medication to prevent organ transplant rejection) causes an increase in tacrolimus blood levels. Therefore, kidney function and tacrolimus blood levels should be frequently monitored.
The concomitant use of metronidazole and amiodarone (a medication for heart arrhythmias) may have an impact on heart activity. Therefore, it is recommended to regularly monitor heart activity using an electrocardiogram (ECG). Consult your doctor immediately if you notice symptoms of heart arrhythmia, such as dizziness, palpitations, or brief fainting spells.
The absorption of mycophenolic acid (a medication that suppresses the immune system) may be reduced when administered concomitantly with medications that alter gastrointestinal flora, such as antibiotics. Therefore, the effect of mycophenolic acid may be reduced when administered concomitantly with metronidazole. Close monitoring of the patient, including laboratory tests, is recommended.
Concomitant administration of metronidazole with phenytoin (used to treat seizures and some types of arrhythmias) or carbamazepine (used to treat seizures) may increase blood levels of phenytoin or carbamazepine, increasing the toxicity of these medications.
Concomitant administration of metronidazole and mebendazole should be avoided, as severe skin reactions (Stevens-Johnson syndrome/toxic epidermal necrolysis) have been reported in patients who received both medications.
Taking Intizol Vaginal with food, drinks, and alcohol
Alcohol consumption should be avoided, as signs of intolerance, such as redness of the skin in the head and neck region, increased heart rate (tachycardia), nausea, vomiting, headache, and dizziness, may appear.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Since metronidazole crosses the placental barrier and there is insufficient data to establish its safety during pregnancy, your doctor will carefully evaluate the convenience of using this medicine during pregnancy.
Metronidazole passes into breast milk, so its administration should be avoided during breastfeeding.
Driving and using machines
This medicine may cause side effects that reduce your ability to drive or use machines. Do not drive or use machines if you experience any of the following symptoms: confusion, dizziness, vertigo (sensation of spinning), hallucinations, seizures/spasms, or vision disorders (such as blurred vision or double vision).
Intizol Vaginal contains soy lecithin
This medicine contains soy lecithin, so you should not use it if you are allergic to soy or peanuts.
3. How to use Intizol Vaginal
Follow the administration instructions for this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one vaginal tablet per day for two consecutive days. It is recommended to administer at night, at bedtime.
Intizol Vaginal is designed to be inserted deeply into the vagina (see illustration).
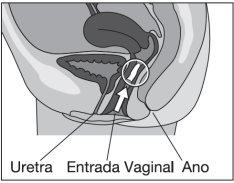
The vaginal tablet is more easily inserted into the vagina while lying on your back with your legs bent.
If you use more Intizol Vaginal than you should
In case of metronidazole overdose, you may experience nausea, vomiting, increased or exaggerated reflexes (hyperreflexia), muscular coordination disorder (ataxia), rapid heart rate (tachycardia), difficulty breathing, and disorientation. There is no specific antidote for this medicine.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Intizol Vaginal
Do not use a double dose to make up for forgotten doses.
If you forget a dose, consult your doctor, as it may be necessary to extend the treatment by one day.
If you stop using Intizol Vaginal
If you stop treatment with this medicine prematurely, consult your doctor, as the treatment may not be effective.
If you have any doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
When using this medicine, approximately 20% of the active ingredient (metronidazole) enters the bloodstream. For this reason, the adverse reactions that occur with the oral administration of metronidazole-containing medications are listed below.
Common side effects(may affect up to 1 in 10 people)
- Decreased appetite
- Metallic taste in the mouth, belching, white tongue, inflammation of the tongue, mouth, or lips, nausea, vomiting, diarrhea
- Abnormal urine color
Uncommon side effects(may affect up to 1 in 100 people)
- Genital fungal infection (Candida)
- Decreased count of some blood cells (leukopenia and granulocytopenia)
- Psychosis, hallucinations, excitability, depression
- Headache, dizziness, sleep disturbances (somnolence and insomnia), coordination disorders (ataxia), seizures, and a nervous disorder with loss of strength and altered sensitivity such as numbness or tingling and, occasionally, pain (peripheral neuropathy)
- Visual disturbances, including optic nerve inflammation (optic neuropathy), double vision, myopia, blurred vision, decreased visual acuity, and changes in color vision
- Itching, skin redness, urticaria
- Muscle weakness
- Difficulty or pain when urinating, bladder inflammation (cystitis), urinary incontinence
- Fever
- Elevated transaminases or bilirubin in blood
Rare side effects(may affect up to 1 in 10,000 people)
- Decreased count of blood cells (agranulocytosis) and platelets (thrombocytopenia)
- Severe allergic reaction (anaphylactic shock). For more information, see Warnings and precautions in section 2.
- Severe and persistent diarrhea (pseudomembranous colitis; for more information, see Warnings and precautions in section 2), pancreatitis
- Joint pain
Side effects with unknown frequency(frequency cannot be estimated from the available data)
- Decreased count of some blood cells (neutropenia)
- Inflammation in the mouth, tongue, face, or throat (angioedema)
- Dizziness, ringing or buzzing in the ears (tinnitus), decreased hearing, and deafness
- Disorder of the nervous system with altered mental status (encephalopathy)
- Disorder of the nervous system with altered speech, altered coordination, and gait, rapid involuntary eye movements, seizures (cerebellar syndrome)
- Aseptic meningitis (a type of inflammation of the brain and spinal cord)
- Hepatitis, yellowing of the skin and eyes (jaundice), liver failure (for more information, see Warnings and precautions in section 2)
- Severe skin reactions that may be associated with fever, fatigue, malaise, and pain (Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, erythema multiforme). Redness and swelling of the skin, rash, blisters, pustules, and skin peeling may occur.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Intizol Vaginal
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date, which is stated on the blister and carton after "EXP". The expiry date is the last day of the month indicated.
Store below 25 °C.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy's SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the containers and any unused medicines. This will help protect the environment.
6. Contents of the pack and other information
Composition of Intizol Vaginal
- The active ingredient is metronidazole. Each vaginal tablet contains 1,000 mg of metronidazole.
- The other ingredients are semi-synthetic solid glycerides with additives and soy lecithin.
Appearance and packaging of the product
Pale yellow, torpedo-shaped vaginal tablets, packaged in blisters and placed in a carton box.
The pack contains 2 vaginal tablets.
Marketing authorization holder
SEID, S.A.
Carretera de Sabadell a Granollers, km 15
08185 Lliçà de Vall, Barcelona, Spain
Manufacturer
Dr. August Wolff GmbH & Co. KG Arzneimittel
Sudbrackstrasse 56
33611 Bielefeld, Germany
Date of the last revision of this leaflet:December 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INTIZOL VAGINAL 1.000 mg VAGINAL SUPPOSITORIESDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 500 mgActive substance: metronidazoleManufacturer: Laboratorios Fidia Farmaceutica S.L.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 100 mg clotrimazoleActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: VAGINAL SEMISOLID, 2% clotrimazole / 100 gActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription required
Online doctors for INTIZOL VAGINAL 1.000 mg VAGINAL SUPPOSITORIES
Discuss questions about INTIZOL VAGINAL 1.000 mg VAGINAL SUPPOSITORIES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






