
INOVELON 40 mg/ml ORAL SUSPENSION

Ask a doctor about a prescription for INOVELON 40 mg/ml ORAL SUSPENSION

How to use INOVELON 40 mg/ml ORAL SUSPENSION
Introduction
Package Leaflet: Information for the User
Inovelon40mg/ml oral suspension
Rufinamide
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. (See section 4).
Contents of the pack and other information:
- What is Inovelon and what is it used for
- What you need to know before you take Inovelon
- How to take Inovelon
- Possible side effects
- Storing Inovelon
- Contents of the pack and other information
1. What is Inovelon and what is it used for
Inovelon contains a medicine called rufinamida. It belongs to a group of medicines called antiepileptics, which are used to treat epilepsy (a disease that causes convulsive seizures or epileptic fits).
Inovelon is used with other medicines to treat seizures associated with Lennox-Gastaut syndrome in adults, adolescents, and children over 1 year of age. Lennox-Gastaut syndrome is the name given to a group of severe epilepsies in which repeated seizures of various types may occur.
Your doctor has prescribed Inovelon to reduce the number of seizures or fits.
2. What you need to know before you take Inovelon
Do not take Inovelon:
- if you are allergic to rufinamide or to triazolic derivatives or to any of the other ingredients of Inovelon (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist:
- if you have congenital short QT syndrome or a family history of this type of syndrome (electrical heart disorder), as the use of rufinamide may worsen it.
- if you have liver problems. The information on the use of rufinamide in this group is limited, so it may be necessary to increase the dose of the medicine more slowly. If your liver disease is severe, the doctor may decide that Inovelon is not recommended for you.
- if you develop a skin rash or fever. They could be signs of an allergic reaction. Go to the doctor immediately, as it can occasionally be severe.
- if you experience an increase in the number or severity or duration of seizures, you should contact your doctor immediately if this happens.
- if you have difficulty walking, abnormal movements, dizziness, or drowsiness, inform your doctor if any of these occur.
- if you take this medicine and have suicidal thoughts or thoughts of self-harm at any time, contact your doctor or go to the hospital immediately(see section 4).
Please consult your doctor, even if you have had these effects at some point in the past.
Children
Inovelon should not be used in children under 1 year of age because there is not enough information about its use in this age group.
Using Inovelon with other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription. If you are using the following medicines: phenobarbital, fosphenytoin, phenytoin, or primidone, you may need to be closely monitored for two weeks at the start or end of treatment with rufinamide, or after any significant change in dose. It may be necessary to change the dose of the other medicines, as they may be less effective when given with rufinamide.
Antiepileptics and Inovelon
If the doctor prescribes or recommends additional treatment for epilepsy (e.g., valproate), you should inform them that you are taking Inovelon, as it may be necessary to adjust the dose.
Taking valproate at the same time as rufinamide in children and adults will result in high levels of rufinamide in the blood. Inform your doctor if you are taking valproate, as it may be necessary to adjust the dose of Inovelon.
Tell your doctor if you are using oral/hormonal contraceptives, i.e., "the pill". Inovelon may make the pill less effective in preventing pregnancy. Therefore, it is recommended that you use another safe and effective contraceptive method (such as a barrier method, e.g., condoms) while using Inovelon.
Tell your doctor if you are using blood thinners, such as warfarin. The doctor may need to adjust the dose.
Tell your doctor if you are using digoxin (a medicine used to treat heart conditions). The doctor may need to adjust the dose.
Taking Inovelon with food and drinks
See section 3 "How to take Inovelon" for recommendations on taking Inovelon with food and drinks.
Pregnancy, breastfeeding, and fertility
If you are pregnant or think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. You should only take Inovelon during pregnancy if your doctor advises you to.
You are advised not to breastfeed while taking Inovelon, as it is not known whether rufinamide passes into breast milk.
If you are a woman of childbearing age, you should use contraceptive methods while taking Inovelon.
Consult your doctor or pharmacist before using any medicine at the same time as Inovelon.
Driving and using machines
Inovelon may make you feel dizzy, drowsy, and affect your vision, especially at the start of treatment or after a dose increase. If this happens, do not drive or use machines.
Inovelon contains sorbitol (E420)
Inovelon contains 175 mg of sorbitol (E420) per milligram. Sorbitol is a source of fructose. If your doctor has told you (or your child) that you have an intolerance to certain sugars, or you have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disease in which the patient cannot break down fructose, consult your doctor before taking this medicine.
Sorbitol may cause gastrointestinal upset and a mild laxative effect.
Taking Inovelon with another antiepileptic medicine that contains sorbitol may affect its functioning. Inform your doctor or pharmacist if you are taking antiepileptic medicines with sorbitol.
Inovelon contains benzoic acid (E210)
Inovelon contains less than 0.01 mg of benzoic acid (E210) per milligram. Benzoic acid may increase the risk of jaundice (yellowing of the skin and eyes) in newborns up to 4 weeks of age.
Inovelon contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per daily dose; this is, essentially "sodium-free".
Inovelon contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate
These components may cause allergic reactions (possibly late).
3. How to take Inovelon
Follow exactly the instructions for administering this medicine as indicated by your doctor. If you are in doubt, consult your doctor or pharmacist again.
Finding the best dose of Inovelon for you may take some time. The doctor will calculate the correct dose based on your age, weight, and whether you are taking Inovelon with another medicine called valproate.
Children between 1 and 4 years old
The recommended starting dose is 10 mg (0.25 ml) per kilogram of body weight per day. It is taken in two equal doses, half in the morning and half in the evening. The doctor will calculate your dose and may increase it in increments of 10 mg (0.25 ml) per kilogram of body weight every three days.
The maximum daily dose will depend on whether you are taking valproate or not. The maximum daily dose without valproate is 45 mg (1.125 ml) per kilogram of body weight per day. The maximum daily dose with valproate is 30 mg (0.75 ml) per kilogram of body weight per day.
Children 4 years or older who weigh less than 30 kg
The recommended starting dose is 200 mg (5 ml) per day. It is taken in two equal doses, half in the morning and half in the evening.
The doctor will calculate your dose and may increase it by 200 mg (5 ml) every three days.
The maximum daily dose will depend on whether you are taking valproate or not. The maximum daily dose without valproate is 1,000 mg (25 ml) per day. The maximum daily dose with valproate is 600 mg (15 ml) per day.
Adults, adolescents, and children who weigh 30 kg or more
The recommended starting dose is 400 mg (10 ml) per day. It is taken in two equal doses, half in the morning and half in the evening.
The doctor will calculate your dose and may increase it by 400 mg (10 ml) every other day.
The maximum daily dose will depend on whether you are taking valproate or not. The maximum daily dose without valproate should not exceed 3,200 mg (80 ml), depending on your body weight. The maximum daily dose with valproate should not exceed 2,200 mg (55 ml), depending on your body weight.
Some patients may respond to lower doses, and the doctor may adjust the dose based on your response to treatment.
If you experience side effects, the doctor may increase the dose more slowly.
Inovelon oral suspension should be taken twice a day, once in the morning and once in the evening. Inovelon should be taken with food.
Method of administration
Use the syringe and adapter provided for administration.
The following instructions for using the syringe and adapter are provided:
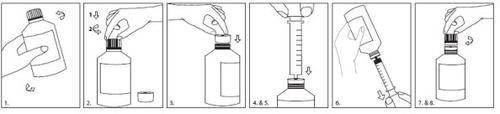
- Shake well before use.
- Press and turn the cap to open the bottle.
- Insert the adapter into the neck of the bottle until it is tightly closed.
- Insert the syringe plunger fully.
- Insert the syringe into the adapter opening as far as possible.
- Turn the bottle upside down and withdraw the prescribed amount of Inovelon.
- Turn the bottle right side up and remove the syringe.
- Leave the adapter in place and replace the cap on the bottle. Wash the syringe with clean water and dry it well.
Do not reduce the dose or stop taking this medicine unless your doctor tells you to.
If you take more Inovelon than you should
If you may have taken more Inovelon than you should, inform your doctor or pharmacist immediately, or contact the emergency department of the nearest hospital, and take the medicine with you.
If you forget to take Inovelon
If you forget to take a dose, continue taking the medicine as usual. Do not take a double dose to make up for the forgotten dose. If you forget to take more than one dose, consult your doctor.
If you stop treatment with Inovelon
If the doctor tells you to stop treatment, follow their instructions regarding the gradual reduction of Inovelon to reduce the risk of an increase in seizures.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Inovelon can cause side effects, although not everybody gets them.
The following side effects may be very serious:
Skin rash and/or fever. They can be signs of an allergic reaction. If this happens, inform your doctor or go to the hospital immediately.
Change in the types of seizures you have/more frequent seizures that last a long time (called status epilepticus). Inform your doctor immediately.
A small number of people being treated with antiepileptics like Inovelon have had suicidal thoughts or thoughts of self-harm. If you have these thoughts at any time, contact your doctor immediately(see section 2).
You may experience the following side effects with this medicine. Inform your doctor if you experience any of the following:
The very common side effects (more than 1 in 10 patients) of Inovelon are:
Dizziness, headache, nausea, vomiting, drowsiness, fatigue.
The common side effects (more than 1 in 100 patients) of Inovelon are:
Nervous system problems, including: difficulty walking, abnormal movements, seizures/convulsions, unusual eye movements, blurred vision, tremors.
Stomach problems, including: stomach pain, constipation, indigestion, soft stools (diarrhea), loss or changes in appetite, weight loss.
Infections: ear infection, flu, nasal congestion, lung infection.
Additionally, patients have experienced: anxiety, insomnia, nosebleeds, acne, skin rash, back pain, infrequent menstruation, bruising, head injuries (as a result of an accidental injury during an epileptic seizure).
The uncommon side effects (between 1 in 100 and 1 in 1,000 patients) of Inovelon are:
Allergic reactions and increased liver function markers (increased liver enzymes).
Reporting side effects
If you experience any side effects, talk to your doctor, even if it is possible side effects that are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Inovelon
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the label of the bottle and on the carton. The expiration date is the last day of the month indicated.
If more than 90 days have passed since the first opening, the remaining suspension in the bottle should not be used.
Do not use the suspension if you notice that the appearance or smell of the medicine has changed. Take the medicine to your pharmacist.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Packaging Contents and Additional Information
Inovelon Composition
- The active ingredient is rufinamide. Each milliliter contains 40 mg of rufinamide. 5 ml contain 200 mg of rufinamide.
- The other components are microcrystalline cellulose and sodium carmellose, anhydrous citric acid, simethicone emulsion 30% (which contains purified water, silicone oil, polysorbate 65, methylcellulose, silica gel, polyethylene glycol stearate, sorbic acid, benzoic acid (E210), and sulfuric acid), poloxamer 188, orange flavor, hydroxyethylcellulose, methylparaben (E218), potassium sorbate (E202), propylparaben, propylene glycol (E1520), sorbitol (E420), non-crystallizing liquid, and purified water.
Product Appearance and Packaging Contents
- Inovelon is a slightly viscous white suspension. It comes in a 460 ml bottle with two identical syringes and a pressure bottle adapter (PIBA). The syringes are graduated in 0.5 ml increments.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Eisai GmbH
Edmund-Rumpler-Straße 3
60549 Frankfurt am Main
Germany
e-mail: [email protected]
Manufacturer:
.
Eisai GmbH
Edmund-Rumpler-Straße 3
60549 Frankfurt am Main
Germany
You can request more information about this medication by contacting the local representative of the Marketing Authorization Holder:
Belgium Eisai SA/NV Tel: +32 (0)800 158 58 | Lithuania Eisai GmbH Tel: + 49 (0) 69 66 58 50 |
Greece Eisai GmbH Tel: + 49 (0) 69 66 58 50 | Luxembourg Eisai SA/NV Tél/Tel: +32 (0)800 158 58 (Belgium) |
Czech Republic Eisai GesmbH organizational unit Tel: + 420 242 485 839 | Hungary Eisai GmbH Tel.: + 49 (0) 69 66 58 50 |
Denmark Eisai AB Tlf: + 46 (0) 8 501 01 600 (Sweden) | Malta Associated Drug Co. Ltd Tel: + 356 2277 8000 |
Germany Eisai GmbH Tel: + 49 (0) 69 66 58 50 | Netherlands Eisai B.V. Tél/Tel: + 31 (0) 900 575 3340 |
Estonia Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) | Norway Eisai AB Tlf: + 46 (0) 8 501 01 600 (Sweden) |
Greece Arriani Pharmaceutical S.A. Τηλ: + 30 210 668 3000 | Austria Eisai GesmbH Tel: + 43 (0) 1 535 1980-0 |
Spain Eisai Farmacéutica, S.A. Tel: + (34) 91 455 94 55 | Poland Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) |
France Eisai SAS Tél: + (33) 1 47 67 00 05 | Portugal Eisai Farmacêutica, Unipessoal Lda Tel: + 351 214 875 540 |
Croatia Eisai GmbH Tel: + 49 (0) 69 66 58 50 | Romania Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) |
Ireland Eisai GmbH Tel: + 49 (0) 69 66 58 50 | Slovenia Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) |
Iceland Eisai AB Sími: + 46 (0)8 501 01 600 (Sweden) | Slovakia Eisai GesmbH organizational unit Tel.: + 420 242 485 839 (Czech Republic) |
Italy Eisai S.r.l. Tel: + 39 02 5181401 | Finland Eisai AB Puh/Tel: + 46 (0) 8 501 01 600 (Sweden) |
Cyprus Arriani Pharmaceuticals S.A. Τηλ: + 30 210 668 3000 (Greece) | Sweden Eisai AB Tel: + 46 (0) 8 501 01 600 |
Latvia Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) | United Kingdom (Northern Ireland) Eisai GmbH Tel: + 49 (0) 69 66 58 50 (Germany) |
Date of Last Revision of this Leaflet:
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INOVELON 40 mg/ml ORAL SUSPENSIONDosage form: TABLET, 100 mgActive substance: rufinamideManufacturer: Eisai GmbhPrescription requiredDosage form: TABLET, 200 mgActive substance: rufinamideManufacturer: Eisai GmbhPrescription requiredDosage form: TABLET, 400 mgActive substance: rufinamideManufacturer: Eisai GmbhPrescription required
Online doctors for INOVELON 40 mg/ml ORAL SUSPENSION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for INOVELON 40 mg/ml ORAL SUSPENSION – subject to medical assessment and local rules.







