How to use HULIO 40 MG/0.8 ML INJECTABLE SOLUTION
Introduction
Package Leaflet: Information for the Patient
Hulio 40 mg/0.8 ml Solution for Injection
adalimumab
This medicinal product is subject to additional monitoring, which will allow quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before your child starts using this medicine, because it contains important information for them.
- Keep this leaflet, you may need to read it again.
- Your doctor will give you a Patient Information Card, which contains important safety information that you need to know before and during your child's treatment with Hulio. Carry the Patient Information Card with you at all times and for 4 months after your child receives the last injection of Hulio.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your child only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as your child's.
- If your child experiences any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Hulio and what is it used for
- What you need to know before your child starts using Hulio
- How to use Hulio
- Possible side effects
- Storage of Hulio
- Contents of the pack and further information
- Instructions for use
1. What is Hulio and what is it used for
Hulio contains the active substance adalimumab, a medicine that acts on the body's immune system (defences).
Hulio is indicated for the treatment of the following inflammatory diseases:
- polyarticular juvenile idiopathic arthritis in children from 2 to 17 years of age;
- enthesitis-related arthritis in children from 6 to 17 years of age;
- paediatric Crohn's disease in children from 6 to 17 years of age;
- plaque psoriasis in children from 4 to 17 years of age;
- hidradenitis suppurativa in adolescents from 12 to 17 years of age;
- non-infectious chronic uveitis affecting the front part of the eye in children from 2 to 17 years of age.
The active substance in Hulio, adalimumab, is a monoclonal antibody. Monoclonal antibodies are proteins that target a specific target in the body.
The target of adalimumab is a protein called tumour necrosis factor (TNFα), which is found at high levels in the inflammatory diseases described above. By targeting TNFα, Hulio reduces the inflammation process in these diseases.
Juvenile idiopathic arthritis and enthesitis-related arthritis
Juvenile idiopathic arthritis and enthesitis-related arthritis are inflammatory diseases of the joints that usually appear for the first time in childhood.
Hulio is used to treat juvenile idiopathic arthritis in children and adolescents from 2 to 17 years of age and enthesitis-related arthritis in children and adolescents from 6 to 17 years of age. Your child may have first received other disease-modifying medicines, such as methotrexate. If these medicines do not respond well enough, your child will receive Hulio to treat their juvenile idiopathic arthritis or enthesitis-related arthritis.
Paediatric Crohn's disease
Crohn's disease is an inflammatory disease of the digestive tract.
Hulio is used to treat Crohn's disease in children from 6 to 17 years of age. Your child will have first received other medicines. If these medicines do not respond well enough, your child will receive Hulio to reduce the signs and symptoms of their Crohn's disease.
Paediatric plaque psoriasis
Plaque psoriasis is an inflammatory disease of the skin that causes red, scaly, crusty, and silvery-scaled areas. Plaque psoriasis can also affect the nails, causing them to deteriorate, thicken, and lift off the nail bed, which can be painful. It is believed that psoriasis is caused by a defect in the body's immune system that leads to an increase in skin cell production.
Hulio is used to treat severe plaque psoriasis in children and adolescents from 4 to 17 years of age who have not responded well to, or are not candidates for, topical therapy and ultraviolet (UV) light therapy.
Hidradenitis suppurativa in adolescents
Hidradenitis suppurativa (also known as inverse acne) is a chronic inflammatory skin disease that is often painful. Symptoms can include painful nodules (lumps) and abscesses (boils) that can secrete pus. It usually affects specific areas of the skin, such as under the breast, in the armpits, inner thighs, groin, and buttocks. There may also be scarring in the affected areas.
Hulio is used to treat hidradenitis suppurativa in adolescents from 12 years of age. Hulio may reduce the number of nodules and abscesses your child has, and the pain that is usually associated with this disease. Patients may have first received other medicines. If these medicines do not respond well enough, patients will receive Hulio.
Non-infectious chronic uveitis affecting the front part of the eye
Non-infectious uveitis is an inflammatory disease that affects certain parts of the eye. This inflammation leads to a decrease in vision and/or the presence of floaters in the eye (black dots or thin lines that move across the field of vision). Hulio works by reducing this inflammation.
Hulio is used in children and adolescents from 2 to 17 years of age for the treatment of chronic non-infectious uveitis with inflammation affecting the front part of the eye.
2. What you need to know before your child starts using Hulio
Do not use Hulio
- If your child is allergic to adalimumab or any of the other ingredients of this medicine (listed in section 6).
- If your child has a severe infection, including tuberculosis (see "Warnings and precautions"). If your child has symptoms of any infection, such as fever, wounds, fatigue, dental problems, it is important that you inform your doctor.
If your child has moderate or severe heart failure. It is important that you tell your doctor if your child has had or has any serious heart problems (see "Warnings and precautions").
Warnings and precautions
Talk to your doctor or pharmacist before starting treatment with Hulio.
Allergic reactions
If you notice in your child an allergic reaction with symptoms such as chest tightness, difficulty breathing, dizziness, swelling, or rash, stop the Hulio injections and contact your doctor immediately, as these reactions can be life-threatening.
Infections
- If your child has an infection, including chronic or localized infections (such as a leg ulcer), consult your child's doctor before starting treatment with Hulio. If you are not sure, contact your child's doctor.
- With treatment with Hulio, your child may be more likely to get infections. This risk may be greater if your child has damaged lungs. These infections can be serious and include tuberculosis, infections caused by viruses, fungi, parasites, or bacteria, or other infectious microorganisms, and sepsis (septicemia), which is rare. In rare cases, these infections can be life-threatening. For this reason, it is important that if your child has symptoms such as fever, wounds, fatigue, or dental problems, you tell the doctor. The doctor may recommend that you temporarily stop treatment with Hulio.
Tuberculosis (TB)
- Since cases of tuberculosis have been reported in patients treated with adalimumab, your child's doctor will examine your child for signs or symptoms of tuberculosis before starting treatment with Hulio. This will include a thorough medical examination, including medical history and diagnostic tests (such as chest X-ray and tuberculin test). The performance and results of these tests must be recorded on your child's Patient Information Card. It is very important that you inform your doctor if your child has had tuberculosis or has been in contact with a tuberculosis patient. Tuberculosis can develop during treatment, even if your child has received preventive treatment for tuberculosis. If symptoms of tuberculosis (persistent cough, weight loss, general malaise, low-grade fever) or any other infection appear during or after treatment, contact your child's doctor immediately.
Recurrent/contracted infection during travel
- Tell your child's doctor if you live in or travel to areas where fungal infections such as histoplasmosis, coccidioidomycosis, or blastomycosis are common.
- Tell your child's doctor if your child has a history of recurrent infections or other conditions or factors that increase the risk of infections.
Hepatitis B virus
- Tell your doctor if your child is a carrier of the hepatitis B virus (HBV), if they have had active HBV infections, or if they think they may be at risk of contracting HBV. Your child's doctor will perform an HBV test on them. Hulio may cause the reactivation of HBV infection in people who carry this virus. In rare cases, especially if your child is taking other medicines that suppress the immune system, the reactivation of HBV infection can be life-threatening.
Surgical or dental intervention
- If your child is going to have surgery or dental treatment, tell your child's doctor that they are taking Hulio. Your child's doctor may recommend that you temporarily stop treatment with Hulio.
Demyelinating disease
- If your child has or develops a demyelinating disease (a disease that affects the protective sheath that surrounds the nerves), such as multiple sclerosis, your child's doctor will decide whether they should be treated or continue treatment with Hulio. Tell the doctor immediately if your child experiences symptoms such as changes in vision, weakness in arms or legs, or numbness or tingling in any part of the body.
Vaccine
- Certain vaccines contain live, attenuated forms of bacteria or viruses that cause diseases and should not be given during treatment with Hulio if they cause infection. Consult with your doctor before your child receives any vaccine. It is recommended that, whenever possible, children receive all scheduled vaccinations for their age before starting treatment with Hulio. If your daughter received Hulio while pregnant, her baby may have a higher risk of infection during the first 5 months after the last dose she received during pregnancy. It is essential that you inform your daughter's doctor and other healthcare professionals about her use of Hulio during pregnancy, so they can decide whether her baby should receive any vaccine.
Heart failure
- It is essential that you tell your child's doctor if your child has had or has any serious heart problems. If your child has mild heart failure and is being treated with Hulio, the doctor must continuously monitor their heart failure. If your child experiences new symptoms of heart failure or worsening of existing symptoms (such as difficulty breathing or swelling of their feet), they should contact their doctor immediately.
Fever, bruising, bleeding, or pale appearance
- In some patients, the body may be unable to produce a sufficient number of the type of blood cells that fight infections (white blood cells) or those that help stop bleeding (platelets). If your child has persistent fever, bruising, or bleeding easily, or is very pale, consult your child's doctor immediately. The doctor may decide to interrupt treatment.
Cancer
- In very rare cases, certain types of cancer have been reported in children and adults treated with adalimumab or other TNFα blockers. People with rheumatoid arthritis of greater severity who have had the disease for a long time may have a higher-than-average risk of developing lymphoma and leukaemia (cancers that affect the blood and bone marrow). If your child is being treated with Hulio, the risk of developing lymphoma, leukaemia, and other types of cancer may increase. A specific and severe type of lymphoma has been observed in some patients treated with Hulio. Some of these patients were also receiving treatment with azathioprine or mercaptopurine. Tell the doctor if your child is taking azathioprine or mercaptopurine with Hulio.
- In addition, cases of non-melanoma skin cancer have been reported in patients using adalimumab. Tell your child's doctor if new skin lesions appear during or after treatment, or if existing lesions change in appearance.
- Cancers other than lymphoma have been reported in patients with a certain lung disease, called chronic obstructive pulmonary disease (COPD), treated with another TNFα blocker. If your child has COPD or is a heavy smoker, they should consult their doctor to determine if treatment with a TNFα blocker is suitable.
Other medicines and Hulio
Tell your doctor or pharmacist if your child is taking, has recently taken, or might take any other medicines.
Hulio can be taken with methotrexate or with certain disease-modifying antirheumatic medicines (sulfasalazine, hydroxychloroquine, leflunomide, and injectable gold preparations), corticosteroids, or pain medicines, including non-steroidal anti-inflammatory drugs (NSAIDs).
Your child must not use Hulio with medicines whose active substances are anakinra or abatacept. Based on the possible increased risk of infections, including serious infections, and other potential pharmacological interactions, the combination of Hulio with anakinra or abatacept is not recommended. If you have any doubts, consult your child's doctor.
Pregnancy and breastfeeding
- Your daughter should consider using adequate contraceptive methods to avoid becoming pregnant and continue using them for at least 5 months after the last treatment with Hulio.
- If your daughter is pregnant, thinks she may be pregnant, or plans to have a baby, ask her doctor for advice before using this medicine.
- Hulio should be used during pregnancy only if necessary.
- According to a pregnancy study, there was no increased risk of congenital malformations when the mother had received treatment with Hulio during pregnancy compared to mothers with the same disease who did not receive treatment with Hulio.
- Hulio can be used during breastfeeding.
- If your daughter uses Hulio during pregnancy, her baby may have a higher risk of infection.
- It is essential that you tell the paediatrician or other healthcare professionals that your daughter uses Hulio during pregnancy before the baby receives any vaccine. For more information on vaccines, see the "Warnings and precautions" section.
Driving and using machines
Hulio has a minor influence on the ability to drive, ride a bicycle, or use machines. Dizziness and vision disturbances may occur after using Hulio.
Hulio contains sodium and sorbitol
Each vial of Hulio contains 38.2 mg of sorbitol. Sorbitol is a source of fructose. If your child's doctor has informed you that your child has intolerance to some sugars or has been diagnosed with hereditary fructose intolerance (HFI), a rare hereditary condition in which the patient cannot break down fructose, talk to your child's doctor before administering the medicine to your child.
In addition, this medicine contains less than 1 mmol of sodium (23 mg) per vial, which is essentially "sodium-free".
3. How to use Hulio
Follow exactly the administration instructions of this medication indicated by your child's doctor or pharmacist. In case of doubt, consult your child's doctor or pharmacist again. Your doctor may prescribe a different dose of Hulio if your child needs a different dose.
Children and adolescents with polyarticular juvenile idiopathic arthritis
Children and adolescents from 2 to 17 years of age with a weight of 10 kg to less than 30 kg:
The recommended dose of Hulio is 20 mg every other week.
Children and adolescents from 2 to 17 years of age with a weight of 30 kg or more:
The recommended dose of Hulio is 40 mg every other week.
Children and adolescents with enthesitis-related arthritis
Children and adolescents from 6 to 17 years of age with a weight of 15 kg to less than 30 kg:
The recommended dose of Hulio is 20 mg every other week.
Children and adolescents from 6 to 17 years of age with a weight of 30 kg or more:
The recommended dose of Hulio is 40 mg every other week.
Children and adolescents with Crohn's disease
Children and adolescents from 6 to 17 years of age with a weight of less than 40 kg:
The usual dosage is 40 mg initially, followed by 20 mg two weeks later. If a faster response is required, the pediatrician may prescribe an initial dose of 80 mg (as two 40 mg injections on the same day) followed by 40 mg two weeks later.
From then on, the usual dose is 20 mg every other week. If this dose does not have the desired effect, the pediatrician may increase the frequency of doses to 20 mg every week.
Children and adolescents from 6 to 17 years of age with a weight of 40 kg or more:
The usual dosage is 80 mg (as two 40 mg injections on the same day) initially, followed by 40 mg two weeks later. If a faster response is required, the pediatrician may prescribe an initial dose of 160 mg (as four 40 mg injections on the same day or two 40 mg injections per day for two consecutive days) followed by 80 mg (as two 40 mg injections on the same day) two weeks later.
From then on, the usual dose is 40 mg every other week. If this dose does not have the desired effect, the pediatrician may increase the dose to 40 mg every week or 80 mg every other week.
Children or adolescents with plaque psoriasis
Children and adolescents from 4 to 17 years of age with a weight of 15 kg to less than 30 kg:
The recommended dose of Hulio is an initial dose of 20 mg, followed by 20 mg one week later. From then on, the usual dose is 20 mg every other week.
Children and adolescents from 4 to 17 years of age with a weight of 30 kg or more:
The recommended dose of Hulio is an initial dose of 40 mg, followed by 40 mg one week later. From then on, the usual dose is 40 mg every other week.
Adolescents with hidradenitis suppurativa (from 12 to 17 years of age, with a weight of at least 30 kg)
The recommended dose of Hulio is an initial dose of 80 mg (as 2 injections of 40 mg on the same day), followed by 40 mg every other week, starting one week later. If this dose does not have the desired effect, the pediatrician may increase it to 40 mg every week or 80 mg every other week.
It is recommended that your child use an antiseptic liquid daily on the affected areas during treatment with Hulio.
Children and adolescents with chronic non-infectious uveitis
Children and adolescents from 2 to 17 years of age with a weight of less than 30 kg:
The usual dose of Hulio is 20 mg every other week, along with methotrexate.
Your pediatrician may prescribe an initial dose of 40 mg, which can be administered one week before starting the recommended usual regimen.
Children and adolescents from 2 to 17 years of age with a weight of 30 kg or more:
The usual dose of Hulio is 40 mg every other week, along with methotrexate.
Your pediatrician may prescribe an initial dose of 80 mg, which can be administered one week before starting the usual regimen.
For patients who are administered a full dose of 40 mg of Hulio, a 40 mg pre-filled pen and a 40 mg pre-filled syringe are also available at the pharmacy.
Form and route of administration
Hulio is injected under the skin (subcutaneous use).
In section 7: Instructions for use, detailed instructions are provided on how to inject Hulio.
If you use more Hulio than you should
If you accidentally inject Hulio to your child at a higher frequency than normal, call your child's doctor or pharmacist and inform them that your child received a higher dose than necessary. Always carry the medicine box with you, even if it is empty.
If you forget to use Hulio
If you forget to administer a dose to your child, you should inject the next dose of Hulio as soon as you remember. Then, the next dose will be administered to your child as usual, as if a dose had not been missed.
If your child stops treatment with Hulio
The decision to stop using Hulio should be discussed with your child's doctor. Your child's symptoms may return after stopping treatment.
If you have any other questions about the use of this medication, ask your child's doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are mild to moderate. However, some can be serious and require urgent medical treatment.
Side effects may appear up to 4 months, or more, after the last injection of Hulio.
Seek medical attention immediatelyif your child has any of the following signs of an allergic reaction or heart failure:
- severe rash, hives;
- swelling of the face, hands, feet;
- difficulty breathing, swallowing;
- pale appearance, dizziness, persistent fever, bruising or bleeding easily.
Contact your doctor as soon as possibleif you notice any of the following effects:
- signs and symptoms of infection such as fever, nausea, wounds, dental problems; burning sensation when urinating, weakness, fatigue or cough;
- symptoms of nervous system problems such as tingling, numbness, double vision or weakness in arms or legs;
- signs of skin cancer, such as a lump or an open sore that does not heal;
- signs and symptoms of blood disorders such as persistent fever, bruising and paleness.
The following side effects have been observed with adalimumab:
Very common(may affect more than 1 in 10 people):
- reactions at the injection site (including pain, swelling, redness or itching);
- lower respiratory tract infections (including colds, runny nose, sinusitis, throat infection, pneumonia);
- abnormal blood values;
- headache;
- abdominal pain;
- nausea and vomiting;
- pain in bones and muscles.
Common(may affect up to 1 in 10 people):
- any infection (including tuberculosis, blood poisoning, flu, cellulitis, herpes, ear infections, dental infections, cold sores, infections of the reproductive system, urinary tract infections, fungal infections);
- benign tumors;
- skin cancer;
- mild allergic reactions (including seasonal allergies);
- dehydration;
- mood changes (including depression);
- anxiety;
- difficulty sleeping;
- sensory disturbances such as tingling, itching or numbness;
- migraine;
- back or neck pain;
- visual disturbances;
- inflammation or swelling of the eyes/lids;
- vertigo (feeling that the room is spinning);
- cough;
- feeling of rapid heartbeat;
- high blood pressure;
- flushing;
- blood clots;
- asthma;
- stomach bleeding;
- indigestion, bloating and heartburn;
- acid reflux;
- dryness in eyes and mouth;
- itching, skin inflammation (including eczema);
- increased sweating;
- hair loss;
- new or worsening psoriasis (red and scaly skin);
- muscle spasms;
- blood in urine;
- kidney problems;
- slow wound healing.
Uncommon(may affect up to 1 in 100 people):
- cancer that affects the lymphatic system (lymphoma);
- immune system disorders that can affect the lungs, skin and lymph nodes (the most common presentation is sarcoidosis);
- inflammation of blood vessels;
- tremors;
- nerve damage;
- stroke;
- double vision;
- hearing loss, ringing in the ears;
- irregular heartbeat;
- lung diseases that can cause difficulty breathing (including inflammation);
- blockage of a pulmonary artery;
- excess fluid around the lung;
- inflammation of the pancreas;
- difficulty swallowing;
- inflammation of the gallbladder, gallstones;
- fatty liver (accumulation of fat in liver cells);
- night sweats;
- scars;
- abnormal muscle crisis;
- systemic lupus erythematosus (including inflammation of the skin, heart, lungs, joints and other organs);
- excessive nocturnal urination;
- impotence.
Rare(may affect up to 1 in 1000 people):
- leukemia (cancer that affects the blood and bone marrow);
- multiple sclerosis;
- nervous system disorders (such as optic neuritis and Guillain-Barré syndrome, which can cause muscle weakness, abnormal sensations, tingling in the arms and upper body);
- heart attack;
- pulmonary fibrosis (scarring in the lungs);
- intestinal perforation/tear;
- inflammation of the liver;
- inflammation of blood vessels in the skin;
- Stevens-Johnson syndrome;
- inflammatory rash on the skin;
- lupus-like syndrome;
- lichenoid reaction on the skin (purple-red rash with itching).
Frequency not known(cannot be estimated from the available data):
- hepatosplenic T-cell lymphoma (a rare blood cancer);
- Merkel cell carcinoma (a type of skin cancer);
- liver failure;
- worsening of a skin rash with muscle weakness.
Reporting of side effects
If your child experiences side effects, consult your child's doctor or pharmacist, even if it is a side effect not listed in this leaflet.
You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hulio
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label/carton after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
Keep the vial in the outer packaging to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Hulio Composition
- The active ingredient is adalimumab
- The other components are: monosodium glutamate, sorbitol, methionine, polysorbate 80, hydrochloric acid, and water for injectable preparations.
Product Appearance and Container Contents
Hulio 40 mg injectable solution (injection) in vials is supplied as a sterile solution of 40 mg of adalimumab dissolved in 0.8 ml of a clear to pale yellowish or slightly opalescent solution.
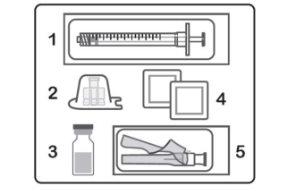
The Hulio vial is a glass vial with a rubber stopper. Hulio is supplied in packs of 1 or 2 boxes. Each box contains 1 vial, 1 sterile syringe for injection, 1 sterile needle, 1 sterile adapter for the vial, and 2 alcohol swabs.
Hulio is available in a pre-filled syringe or pre-filled pen.
Marketing Authorization Holder
Mylan S.A.S.
117 allée des Parcs
69800 Saint-Priest
France
Manufacturer
AndersonBrecon (UK) Limited
Wye Valley Business Park
Brecon Road
Hay-on-Wye
Hereford
HR3 5PG
United Kingdom
McDermott laboratories T/A Mylan Dublin Biologics
Newenham Court, Northern Cross, Malahide Road
Dublin 17
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Mylan EPD bvba/sprl Tél/Tel: + 32 (0)2 658 61 00 | Lietuva BGP Products UAB Tel.: +370 5 205 1288 |
България Милан фарма ЕООД Тел.: +359 2 44 55 400 | Luxemburgo/Luxemburg Mylan EPD bvba/sprl Tel.: + 32 (0)2 658 61 00 (Belgique/Belgien) |
Česká republika Mylan Pharmaceuticals.s.r.o. Tel.: + 420 222 004 400 | Magyarország Mylan EPD Kft Tel.: + 36 1 465 2100 |
Danmark BGP Products ApS Tlf: +45 28116932 | Malta V.J. Salomone Pharma Ltd Tel.: + 356 21 22 01 74 |
Deutschland Mylan dura GmbH Tel.: + 49-(0) 6172 888 01 | Nederland Mylan BV Tel.: +31 (0)20 426 3300 |
Eesti BGP Products Switzerland GmbH Eesti filiaal Tel.: + 372 6363 052 | Norge Mylan AB Tel.: + 46 855 522 750 (Sverige) |
Ελλάδα BGP ΠΡΟ?ΟΝΤΑ Μ.Ε.Π.Ε. Τηλ.: +30 210 9891 777 España Mylan Pharmaceuticals, S.L Tel.: + 34 900 102 712 | Österreich Arcana Arzneimittel GmbH Tel.: +43 1 416 2418 Polska Mylan Healthcare Sp. z.o.o. Tel.: + 48 22 546 64 00 |
France Mylan Medical S.A.S Tel: +33 1 56 64 10 70 | Portugal Mylan, Lda. Tel.: + 351 21 412 72 56 |
Hrvatska Mylan Hrvatska d.o.o. Tel.: +385 1 23 50 599 | România BGP Products SRL Tel: + 40 372 579 000 |
Ireland Mylan Ireland Limited Tel.: +353 (0) 87 1694982 | Slovenija GSP Proizvodi d.o.o. Tel.: + 386 1 236 31 85 |
Ísland BGP Products ApS Sími: +45 28116932 (Danmörk) | Slovenská republika Mylan s.r.o. Tel.: +421 2 32 199 100 |
Italia Mylan S.p.A Tel.: + 39 02 612 46921 | Suomi/Finland Mylan Finland Oy Puh/Tel: +358 20 720 9555 |
Κύπρος Pharmaceutical Trading Co. Ltd. Τηλ: + 357 99403969 | Sverige Mylan AB Tel.: + 46 855 522 750 |
Latvija BGP Products SIA Tel.: +371 676 055 80 | United Kingdom Generics [UK] Ltd Tel.: +44 1707 853000 |
Date of Last Revision of this Prospectus {MM/YYYY}
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Instructions for Use
Read the instructions carefully and follow them step by step. Your child's doctor, a nurse, or another healthcare professional will show you how to prepare the injection and administer it to your child. They will also indicate the prescribed amount (volume).
Do not attempt to give your child the injection before you are sure you understand how to do it. After a suitable training period, you can self-inject or another person can administer the injection, for example, a family member or caregiver.
Each vial contains a dose of 40 mg of adalimumab.
Do not mix any other medication in the same syringe or vial with the Hulio solution.
It may be helpful to make notes on a calendar or diary to remember which days you should inject Hulio.
Before Starting
Make sure you know the prescribed amount. If you do not know it, STOP HERE and consult your child's doctor.
Find a quiet place with a well-lit, clean, and flat work surface and gather all the supplies you will need to apply the injection.
Supplies you will need:
- 1 box of Hulio vials for pediatric use
- 1 container for disposing of sharp objects (not included in the Hulio box)
- 1 gauze or cotton ball (not included in the Hulio box)
If you do not have all the necessary supplies, ask the nursing staff or pharmacist for them.
Preparing the Hulio Injection Each box of Hulio vials contains:
|
|
Hulio packs should be stored in the refrigerator (between 2 and 8 °C) until they are needed.
- Remove one box from the refrigerator at least 30 minutes before use to allow the contents to reach room temperature. If there is a second box in the Hulio pack for a future injection, put it back in the refrigerator immediately.
- ? DO NOT use any heat source, such as a microwave oven or hot water to warm the vial.
- ? DO NOT put the vial back in the refrigerator once it has reached room temperature.
- Check the expiration date printed on the vial label.
- ? DO NOT use the vial after the expiration date.
- Check that the solution in the vial is clear, colorless, and free of particles.
- ? DO NOT use the vial if the solution is cloudy, white, or contains particles in suspension.
Injection Steps
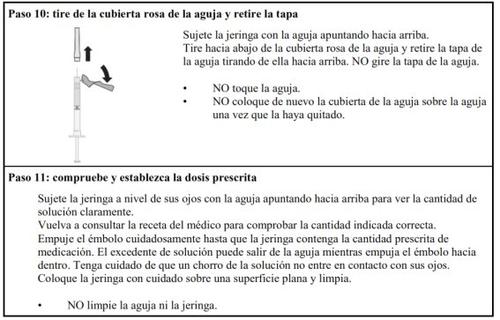
Follow the steps below carefully each time you inject Hulio:

Preparing the Hulio Injectable Dose
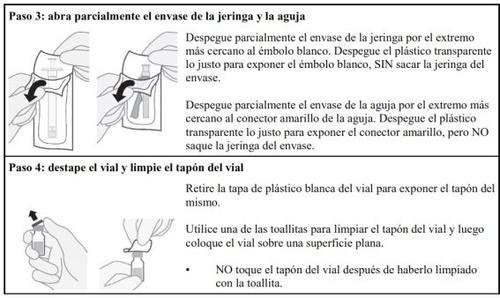
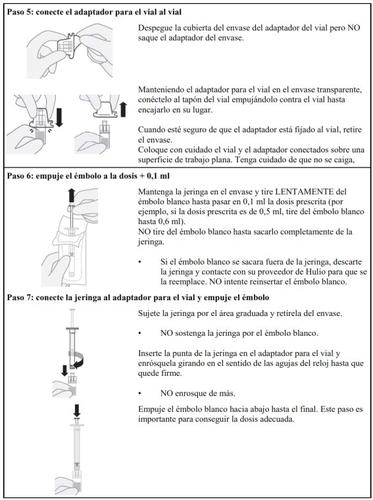

Dose Preparation
Hulio Injection
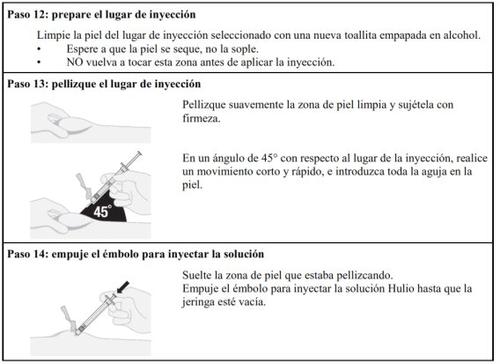

Disposal of Materials

- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HULIO 40 MG/0.8 ML INJECTABLE SOLUTIONDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 40 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription required
Alternatives to HULIO 40 MG/0.8 ML INJECTABLE SOLUTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to HULIO 40 MG/0.8 ML INJECTABLE SOLUTION in Ukraina
Online doctors for HULIO 40 MG/0.8 ML INJECTABLE SOLUTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for HULIO 40 MG/0.8 ML INJECTABLE SOLUTION – subject to medical assessment and local rules.