
GLUCAGEN HYPOKIT 1 mg POWDER AND SOLVENT FOR INJECTION


How to use GLUCAGEN HYPOKIT 1 mg POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
GlucaGen Hypokit 1mg
Powder and solvent for solution for injection
glucagon
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What GlucaGen HypoKit is and what it is used for
- What you need to know before you use GlucaGen HypoKit
- How to use GlucaGen HypoKit
- Possible side effects
- Storage of GlucaGen HypoKit
- Contents of the pack and other information
- Further information for healthcare professionals
1. What GlucaGen HypoKit is and what it is used for
GlucaGen HypoKit contains the active substance “glucagon”.
GlucaGen HypoKit is used immediately in emergency situations in children and adults with diabetes who use insulin. It is used when they have fainted (are unconscious) due to a very low blood sugar level. This is called “severe hypoglycaemia”. GlucaGen HypoKit is used when they are unable to take sugar by mouth.
Glucagon is a natural hormone that has the opposite effect of insulin in the human body. It helps the liver to convert something called “glycogen” into glucose (sugar). The glucose is released into the bloodstream, which makes the blood sugar level rise.
For healthcare professionals:see section 7.
2. What you need to know before you use GlucaGen Hypokit
Important information
- Make sure that your family members, the people you work with, or close friends know about GlucaGen HypoKit. Tell them that if you faint (become unconscious), they should use GlucaGen HypoKit immediately.
- Show your family members and other people where you keep this kit and how to use it. They should act quickly - if you are unconscious for a period of time, it can be harmful. It is essential that they are trained and know how to use GlucaGen HypoKit before you need it.
- The syringe does not contain GlucaGen. The water in the syringe must be mixed with the compacted GlucaGen powder in the vial before injection. Tell your family members and other people to follow the instructions in section 3: How to use GlucaGen HypoKit.
- Any mixture of GlucaGen that is not used should be discarded.
- After using GlucaGen HypoKit, you or another person should contact your doctor or a healthcare professional. You need to find out why you had low blood sugar and how to avoid it happening again.
Do not use GlucaGen HypoKit if
- you are allergic to glucagon or any of the other ingredients of this medicine (listed in section 6).
- you have an adrenal gland tumour.
If any of these apply to you, do not use GlucaGen HypoKit.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting to use GlucaGen Hypokit.
GlucaGen will not work properly if:
- you have been fasting for a prolonged period
- you have low levels of adrenaline
- you have low blood sugar caused by drinking too much alcohol
- you have a tumour that releases glucagon or insulin
If any of these apply to you, talk to your doctor or nurse.
Using GlucaGen with other medicines
The following medicines may affect how GlucaGen HypoKit works:
- insulin – used to treat diabetes
- indometacin – used to treat pain and stiffness in the joints
The following medicines may be affected by GlucaGen HypoKit:
- warfarin – used to prevent blood clots. GlucaGen may increase the anticoagulant effect of warfarin.
- beta-blockers – used to treat high blood pressure and irregular heartbeat. GlucaGen HypoKit may increase blood pressure and pulse, which will only last for a short time.
If any of these apply to you (or you are not sure), talk to your doctor or pharmacist before using GlucaGen HypoKit.
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you experience low blood sugar when you are pregnant or breastfeeding, think you might be pregnant, or plan to become pregnant, you can use GlucaGen HypoKit.
Talk to your doctor or pharmacist before using any medicine if you are pregnant.
Driving and using machines
Wait until the effects of low blood sugar have gone before driving or using tools or machines.
GlucaGen contains sodium
GlucaGen contains less than 23 mg of sodium (1 mmol) per maximum dose (2 ml), i.e., it is essentially “sodium-free”.
3. How to use GlucaGen HypoKit
Follow the instructions for administering the medicine contained in this leaflet or as indicated by your doctor. In case of doubt, ask your doctor.
Preparation and administration of the injection
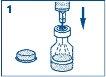
- Remove the plastic cap from the vial. Remove the needle protector from the syringe. Do not remove the plastic safety device from the syringe. Insert the needle into the rubber disc (inside the marked circle) of the vial containing GlucaGen and inject all the liquid from the syringe into the vial.
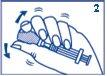
- Without removing the needle from the vial, gently shake the vial until GlucaGen is completely dissolved and the solution is clear.
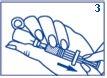
- Make sure the plunger is fully in. While keeping the needle in the liquid, slowly pull out all the solution with the syringe. Try to keep the plunger from coming out of the syringe. It is essential to remove any air bubbles from the syringe:
- With the needle pointing upwards, gently tap the syringe with your fingers
- Press the plunger slightly to release any air bubbles that may have remained at the top of the syringe.
Continue pressing the plunger until you have the correct dose for injection. A small amount of liquid will come out of the needle tip when you do this.
See below Dose to be injected
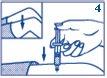
- Inject the dose under the skin or into the muscle.
- Place the unconscious person on their side to prevent choking.
- Give the person a sugary snack such as sweets, biscuits, or fruit juice as soon as they regain consciousness and are able to swallow. The sugary snack will prevent low blood sugar from happening again.
After using GlucaGen HypoKit, you or another person should contact your doctor or a healthcare professional. You need to find out why you had low blood sugar and how to avoid it happening again.
Dose to be injected
The recommended dose is:
- Adults: inject the entire medicine (1 ml) – this is marked as “1” on the syringe.
- Children under 8 years or children over 8 years with a weight below 25 kg: Inject half of the medicine (0.5 ml) – this is marked as “0.5” on the syringe.
- Children over 8 years or children under 8 years with a weight above 25 kg: Inject the entire medicine (1 ml) – this is marked as “1” on the syringe.
If you are given too much GlucaGen
Too much GlucaGen may cause nausea and vomiting. Normally, no specific treatment is needed.
In case of overdose or accidental ingestion, talk to your doctor or pharmacist immediately or call the Toxicological Information Service, telephone (91) 562.04.20, indicating the medicine and the amount ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur with this medicine:
Tell your doctor immediately if you experience any of the following serious side effects:
Very rare:may affect up to 1 in 10,000 people
- Allergic reaction – symptoms can include difficulty breathing, sweating, rapid heartbeat (tachycardia), skin rash, swelling of the face, and collapse.
Tell your doctor immediatelyif you experience any of the above side effects.
Other side effects
Common:may affect up to 1 in 10 people
- feeling sick (nausea)
Uncommon:may affect up to 1 in 100 people
- vomiting
Rare:may affect up to 1 in 1,000 people
- stomach pain (abdominal)
Frequency not known:frequency cannot be estimated from the available data
- reactions at the injection site.
If you experience any of the above side effects, talk to your doctor, even if it is a possible side effect not listed in this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if it is a possible side effect not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of GlucaGen Hypokit
- Keep this medicine out of the sight and reach of children
- Store:
- in the refrigerator(between 2°C and 8°C), or
- outside the refrigerator, below 25°C for up to 18 months, within the validity period.
- Store in the original packaging to protect from light
- Do not freeze to prevent damage to the product
- Use immediately after mixing. Do not store it for later use
- Do not use this medicine after the expiry date which is stated on the label. The expiry date is the last day of the month shown.
- Do not use if the mixed solution looks like gel or if part of the powder has not dissolved properly.
- Do not use if the plastic cap is missing or loose when you receive the product. In this case, return the product to your pharmacy.
- Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of GlucaGen
- The active substance is glucagon 1 mg as hydrochloride, produced in yeast by recombinant DNA technology.
- The other ingredients are: lactose monohydrate, water for injections, hydrochloric acid, and/or sodium hydroxide (for pH adjustment).
Appearance of the product and contents of the pack
GlucaGen is presented in a vial with glucagon, white and sterile powder, with a disposable syringe containing the solvent. The powder is compacted. Once mixed, the reconstituted solution contains glucagon 1 mg/ml.
Marketing authorisation holder and manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd, Denmark
This medicine is authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Croatia, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Hungary, Ireland, Iceland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Portugal, United Kingdom (Northern Ireland), Czech Republic: GlucaGen
Norway and Sweden: Glucagon Novo Nordisk
- Further information for healthcare professionals
Healthcare professionals should consult all the previous sections before reading this additional information.
Due to the instability of GlucaGen in solution, the product should be administered immediately after reconstitution and should not be administered as an intravenous infusion.
Do not attempt to put the cap back on the used syringe needle. Place the used syringe in the orange container and dispose of the used needle in a sharps container when you have the opportunity.
Treatment of severe hypoglycaemia
Administer as a subcutaneous or intramuscular injection. If the patient does not respond within 10 minutes, intravenous glucose should be administered. When the patient has responded to treatment, oral carbohydrates should be administered to restore hepatic glycogen and prevent recurrence of hypoglycaemia.
Diagnostic procedures
Oral carbohydrates should be administered when the procedure is completed, if this is compatible with the diagnostic procedure used. Remember that GlucaGen has the opposite effect to insulin. In endoscopic or radiographic procedures, caution should be exercised when administering GlucaGen to diabetic patients or people with heart problems.
In diagnostic procedures, it may be more appropriate to use a syringe with a finer needle and more precise graduation.
Examination of the gastrointestinal tract:
Doses vary from 0.2 – 2 mg depending on the diagnostic technique used and the route of administration. The diagnostic dose to produce relaxation of the stomach, duodenal bulb, duodenum, and small intestine is 0.2 – 0.5 mg administered intravenously or 1 mg intramuscularly. The dose to relax the colon is 0.5 – 0.75 mg intravenously or 1 – 2 mg intramuscularly. The onset of effect after intravenous injection of 0.2 – 0.5 mg occurs within 1 minute and the duration of effect is between 5 – 20 minutes. The onset of effect after intramuscular injection of 1 – 2 mg occurs after 5 – 15 minutes and lasts approximately 10 – 40 minutes.
Additional side effects after use in diagnostic procedures
Changes in blood pressure, rapid heartbeat, hypoglycaemia, and hypoglycaemic coma.
Date of last revision of this leaflet: 12/2022
Other sources of information
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price21.46 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GLUCAGEN HYPOKIT 1 mg POWDER AND SOLVENT FOR INJECTIONDosage form: NASAL PRODUCT, 3 mgActive substance: glucagonManufacturer: Amphastar France PharmaceuticalsPrescription required
Online doctors for GLUCAGEN HYPOKIT 1 mg POWDER AND SOLVENT FOR INJECTION
Discuss questions about GLUCAGEN HYPOKIT 1 mg POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











