FYDRANE 0.2 mg/mL + 3.1 mg/mL + 10 mg/mL Injectable Solution


How to use FYDRANE 0.2 mg/mL + 3.1 mg/mL + 10 mg/mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
FYDRANE0.2 mg/ml + 3.1 mg/ml + 10 mg/ml, injectable solution
tropicamide / phenylephrine hydrochloride / lidocaine hydrochloride monohydrate
Read the entire package leaflet carefully before starting to use the medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is FYDRANE and what is it used for
- What you need to know before starting to use FYDRANE
- How to use FYDRANE
- Possible side effects
- Storage of FYDRANE
- Package Contents and Additional Information
1. What is FYDRANE and what is it used for
What is FYDRANE
This medicine is a solution for injection into the eye.
It contains three active substances:
- Tropicamide, which belongs to a group of medicines that block the passage of impulses through nerves (known as anticholinergics),
- Phenylephrine (as phenylephrine hydrochloride) which belongs to a group of medicines that mimic the effects of impulses transmitted through certain nerves (which belong to alpha-sympathomimetics),
- Lidocaine (as lidocaine hydrochloride monohydrate) which belongs to a group of medicines called local anesthetics of the amide type.
What it is used for
This medicine is used only in adults.
It will be administered by your ophthalmic surgeon through an injection into the eye at the start of cataract surgery (opacity of the lens) to dilate the pupil of your eye (mydriasis) and obtain anesthesia in your eye during the surgical procedure.
2. What you need to know before starting to use FYDRANE
FYDRANE should not be administered to you:
- if you are allergic to tropicamide, phenylephrine hydrochloride, and/or lidocaine hydrochloride monohydrate or to any of the other components of this medicine (listed in section 6),
- if you are allergic to amide-type anesthetics (such as articaine, bupivacaine, mepivacaine, prilocaine, ropivacaine),
- if you are allergic to atropine derivatives.
Warnings and Precautions
FYDRANE is not recommended:
- in cataract surgery combined with a certain type of eye surgery (vitrectomy),
- if the anterior part (anterior chamber) of your eye is shallow,
- if you have ever suffered from an acute increase in eye pressure (acute angle-closure glaucoma).
Consult your doctor if you have:
- high blood pressure (hypertension)
- thickening of the arterial walls (arteriosclerosis)
- any heart disease, and particularly if it affects heart rate,
- a contraindication to medications that increase blood pressure (vasopressor amines: epinephrine, norepinephrine, dopamine, dobutamine) by systemic route,
- overactive thyroid gland (hyperthyroidism),
- prostate gland disorders,
- seizures (epilepsy),
- any liver or kidney disease,
- any respiratory problems,
- muscle weakness and loss of muscle function (myasthenia gravis).
Using FYDRANE with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Pregnancy, Breastfeeding, and Fertility
This medicine should not be used:
- during pregnancy
- during breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and Using Machines
FYDRANE has a moderate influence on the ability to drive and use machines. Therefore, you should not drive and/or use machines until your vision is normal.
FYDRANE contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially "sodium-free".
3. How to use FYDRANE
This medicine should only be administered if you have already demonstrated, in a preoperative evaluation, satisfactory pupil dilation with a topical mydriatic.
Dose and Method of Administration
- FYDRANE injectable solution should be administered by an ophthalmic surgeon, with local anesthesia, at the start of cataract surgery.
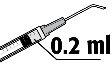
- The recommended dose is 0.2 ml of solution in a single injection. No additional doses should be injected since no cumulative effects have been demonstrated and because an increase in endothelial cell loss has been observed.
- The same dose is used in adults and in elderly patients.
If you are given too much or too little FYDRANE:
Your medication will be administered by an ophthalmic surgeon. It is unlikely that you will be given an overdose. An overdose may cause loss of corneal endothelial cells (cells of a layer that covers the posterior surface of the cornea).
If you have any further questions about the use of this product, ask your doctor, pharmacist, or nurse.
4. Possible Side Effects
Like all medicines, this medicine can have side effects, although not everyone gets them.
The most serious known complications occur during or after cataract surgery:
Uncommon: may affect up to 1 in 100 people
- Damage to the lens (posterior capsule rupture),
- Inflammation of the retina (cystoid macular edema).
Please seek urgent medical attention in these cases.
Other side effects:
Uncommon: may affect up to 1 in 100 people
- Headache,
- Inflammation of the cornea (keratitis), increased pressure in the eye, redness of the eye (ocular hyperemia),
- High blood pressure (hypertension).
Reporting Side Effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of FYDRANE
Keep out of sight and reach of children.
Do not use this medicine after the expiration date stated on the carton, blister, and ampoule after "EXP.". The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
For use in a single eye. This medicine should be used immediately after opening the ampoule.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Composition of FYDRANE
- The active substances are tropicamide 0.04 mg, phenylephrine hydrochloride 0.62 mg, and lidocaine hydrochloride monohydrate 2 mg per 0.2 ml dose, equivalent to 0.2 mg of tropicamide, 3.1 mg of phenylephrine hydrochloride, and 10 mg of lidocaine hydrochloride monohydrate per 1 ml.
- The other components are: sodium chloride, disodium phosphate dodecahydrate, disodium phosphate dihydrate, disodium edetate, and water for injectable preparations.
Appearance and Packaging of the Product
FYDRANE is a clear, yellow to slightly brownish injectable solution, practically free of visible particles, packaged in a 1 ml topaz glass ampoule. Each sterile ampoule contains 0.6 ml of the injectable solution and is presented alone or with a sterile 5-micron filter needle in a sealed paper/PVC blister.
Each package contains 1, 20, or 100 sterile ampoules (with a sterile 5-micron filter needle). The 5-micron filter needle should only be used to extract the contents of the vial.
All components are for single use only.
Not all package sizes may be marketed.
Marketing Authorization Holder
LABORATOIRES THEA
RUE LOUIS BLÉRIOT, 12
F-63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Manufacturer
DELPHARM TOURS
RUE PAUL LANGEVIN
37170 CHAMBRAY LES TOURS
FRANCE
OR
LABORATOIRES THEA
RUE LOUIS BLÉRIOT, 12
F-63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Local Representative:
LABORATORIOS THEA, S.A.
C/ Enric Granados, nº 86-88, 2ª planta
08008 Barcelona
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Bulgaria, Cyprus, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Italy, Luxembourg, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden, United Kingdom ………………………………. Mydrane
Ireland, Spain …………………………………………. Fydrane
Norway ………………………………………… Mydane
Date of the last revision of this package leaflet: September 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.es/
This information is intended only for healthcare professionals:
Incompatibilities
No incompatibilities of the active substances with most products used in cataract surgery have been reported in the literature, and none were found during clinical trials. For usual viscoelastics, this has also been confirmed by pharmacological interaction tests.
Warnings
Do not use if the blister is damaged or broken. Open only under aseptic conditions to ensure the sterility of the contents.
How to Prepare and Administer FYDRANE
Single use of the solution for one eye by intracameral route only.
FYDRANE should be administered by intracameral injection into the anterior chamber of the eye, by an ophthalmic surgeon, under aseptic conditions recommended for cataract surgery.
Before intracameral injection, the solution should be visually inspected and only used if it is a clear solution with a slight yellow to slightly brownish color and practically free of visible particles.
The recommended dose is 0.2 ml of FYDRANE; no additional dose should be injected since no significant cumulative effects have been demonstrated and because an increase in endothelial cell loss has been observed.
The product should be used immediately after opening the ampoule and should not be reused for the other eye or for any other patient.
Only for the kit presentation (i.e., blister containing an ampoule and a needle): attach the detachable label from the blister to the patient's medical history.
To prepare FYDRANE for intracameral administration, follow these instructions: | |
|
|
After use, properly dispose of the remaining solution. It should not be kept for future use. |
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations. Dispose of used needles in a puncture-proof container.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FYDRANE 0.2 mg/mL + 3.1 mg/mL + 10 mg/mL Injectable SolutionDosage form: OPHTHALMIC IMPLANT, 5.376mg Phenylephrine Hydrochloride/0.28mg TropicamideActive substance: tropicamide, combinationsManufacturer: Laboratoires TheaPrescription requiredDosage form: EYEDROP, -Active substance: atropineManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 5 mgActive substance: atropineManufacturer: Alcon Healthcare S.A.Prescription required
Online doctors for FYDRANE 0.2 mg/mL + 3.1 mg/mL + 10 mg/mL Injectable Solution
Discuss questions about FYDRANE 0.2 mg/mL + 3.1 mg/mL + 10 mg/mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions