
FOTIVDA 1340 micrograms hard capsules

How to use FOTIVDA 1340 micrograms hard capsules
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Fotivda 890micrograms hard capsules
Fotivda 1340micrograms hard capsules
tivozanib
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fotivda and what is it used for
- What you need to know before you take Fotivda
- How to take Fotivda
- Possible side effects
- Storing Fotivda
- Contents of the pack and other information
1. What is Fotivda and what is it used for
The active substance in Fotivda is tivozanib, which is a protein kinase inhibitor. Tivozanib reduces the blood supply to the cancer, which delays the growth and spread of cancer cells. It works by blocking the action of a protein called vascular endothelial growth factor (VEGF). Blocking the action of VEGF prevents the formation of new blood vessels.
Fotivda is used to treat adults with advanced kidney cancer. It is used when other treatments such as interferon-alpha or interleukin-2 have not been used yet or have not been effective in stopping your disease.
2. What you need to know before you take Fotivda
Do not take Fotivda:
- If you are allergic to tivozanib or any of the other ingredients of this medicine (listed in section 6).
- If you are taking St. John's Wort (also known as Hypericum perforatum, a herbal medicine used to treat depression and anxiety).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start taking Fotivda:
- If you have high blood pressure.
Fotivda may increase your blood pressure. Your doctor will check your blood pressure regularly and, if it is too high, may give you a medicine to lower it or decide to reduce your dose of Fotivda. However, if your blood pressure remains too high, your doctor may decide to stop or interrupt treatment with Fotivda. If you are already taking a medicine to treat high blood pressure and your doctor reduces your dose of Fotivda or stops or interrupts treatment, you will be monitored regularly for low blood pressure.
- If you have or have had an aneurysm (enlargement and weakening of a blood vessel wall) or a tear in the wall of a blood vessel.
- If you have had blood clot problems.
Treatment with Fotivda may increase the risk of developing a blood clot (thrombosis) in your blood vessels that could break loose and be carried by the bloodstream to block another blood vessel. Tell your doctor if you have ever had any of the following problems:
- a blood clot in the lungs (with cough, chest pain, sudden shortness of breath, or blood in the cough)
- a blood clot in the legs or arms, eye, or brain (with pain or swelling of the hands or feet, vision loss, or changes in mental status)
- a stroke or signs and symptoms of a 'mini-stroke' (transient ischemic attack)
- a heart attack
- high blood pressure
- diabetes
- major surgery
- multiple injuries, such as broken bones and damage to internal organs
- inability to move for a long period
- heart failure that can cause shortness of breath or swelling of the ankles
- inability to breathe, bluish discoloration of the skin, fingertips, or lips, restlessness, anxiety, confusion, altered consciousness, rapid and shallow breathing, rapid heartbeat, or excessive sweating
- If you have or have been treated for any of these symptoms or are being treated for heart failure:
- Shortness of breath (dyspnea) when exercising or when lying down
- Feeling weak and tired
- Swelling (edema) of the legs, ankles, and feet
- Reduced ability to exercise
- Persistent cough or wheezing with white or pink-tinged phlegm
Your doctor will monitor the signs and symptoms of heart failure while you are taking your medicine. If necessary, your doctor may reduce your dose of Fotivda or stop or interrupt treatment.
- If you have or have been treated for an abnormal heart rhythm (arrhythmia).Your doctor will monitor the effect of Fotivda on your heart by recording the electrical activity (electrocardiogram) or measuring the levels of calcium, magnesium, and potassium in your blood during treatment.
- If you have liver problems.
Your doctor will regularly check how well your liver is working before and during treatment with Fotivda (e.g., with blood tests) and, if necessary, may need to reduce the frequency of taking Fotivda.
- If you have thyroid problemsor are taking medicines to treat thyroid disease.Treatment with Fotivda may make your thyroid gland work less well than usual. Your doctor will regularly check how well your thyroid gland is working before and during treatment with Fotivda (e.g., with blood tests).
Talk to your doctor, pharmacist, or nurse while taking Fotivda:
- If you have shortness of breath or swelling of the ankles.Tell your doctor immediately, as these may be symptoms of heart failure. Your doctor will monitor you and, depending on the severity, may reduce your dose of Fotivda or stop or interrupt treatment with Fotivda.
- If you have had bleeding problems.Treatment with Fotivda may increase the risk of bleeding. If you develop bleeding problems (with painful swelling of the stomach, vomiting blood, coughing up blood, black stools, blood in the urine, headache, or changes in mental status), tell your doctor immediately. It may be necessary to temporarily stop treatment with Fotivda.
- If laboratory tests show that there are proteins in the urine.Your doctor will monitor you at the start and during your treatment. Depending on the results, your doctor may reduce your dose of Fotivda or stop or interrupt treatment.
- If you have a brain disease called posterior reversible encephalopathy syndrome (PRES).Tell your doctor immediately if you have symptoms such as headache, seizures, lack of energy, confusion, blindness, or other visual and neurological disturbances such as weakness in an arm or leg. If you are diagnosed with PRES, your doctor will stop treatment with Fotivda.
- If the skin on the palms of your hands and soles of your feetbecomes dry, cracked, scaly, or peeling or has itching or tingling
- These could be symptoms of a problem called hand-foot skin reaction. Your doctor will treat the problem and, depending on the severity, may reduce your dose of Fotivda or stop or interrupt treatment with Fotivda.
- If you have symptoms of gastrointestinal perforation or fistula formation(developing a hole in the stomach or intestine or formation of abnormal passages between parts of the intestine) such as severe stomach pain, chills, fever, nausea, vomiting, or painful intestinal blockage, diarrhea, or rectal bleeding.
Your doctor will regularly monitor you for these symptoms during your treatment with Fotivda.
- If you need to have an operation or other surgery.Your doctor may recommend that you temporarily stop taking Fotivda if you are going to have an operation or surgery, as it may affect wound healing.
Children and adolescents
Do notgive Fotivda to children and adolescents under 18 years. This medicine has not been studied in children and adolescents.
Other medicines and Fotivda
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This includes herbal medicines and other medicines that you have bought without a prescription.
Fotivda may not work as well when taken with some medicines. Tell your doctor if you are taking any of the following medicines; they may decide to change your medication:
- dexamethasone (a corticosteroid to reduce inflammation and treat immune system disorders);
- rosuvastatin (a medicine used to help lower blood cholesterol levels);
- phenobarbital, phenytoin, carbamazepine (used to treat epilepsy);
- nafcillin, rifampicin, rifabutin, rifapentine (antibiotics);
- St. John's Wort (also known as Hypericum perforatum, a herbal medicine used to treat depression and anxiety), as this herbal medicine must not be taken at the same time as Fotivda.
Pregnancy, breast-feeding, and fertility
- Do not take Fotivda if you are pregnant.Tell your doctor, who will discuss the risks of taking Fotivda for you and your baby.
- Both you and your partner must use effective contraception.If you or your partner are taking hormonal contraceptives (the pill, an implant, or a patch) you must also use a barrier methodthroughout treatment and for another month after finishing treatment.
- Stop breast-feeding during treatment with Fotivda, as it is not known whether the active substance in Fotivda passes into breast milk. Talk to your doctor if you are already breast-feeding your baby.
- Talk to your doctor if you plan to have a baby, as Fotivda may affect fertilityin males and females.
Driving and using machines
Fotivda may have side effects that could affect your ability to drive or use machines. Do not drive or use machines if you feel weak, tired, or dizzy. See also section 4 "Possible side effects".
Fotivda contains tartrazine (E102)
The printing ink used for the Fotivda 890 micrograms capsule contains tartrazine (E102), which may cause allergic reactions.
3. How to take Fotivda
Take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Recommended dose
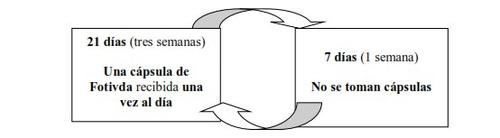
The recommended dose is one Fotivda 1340 micrograms capsule, taken once daily for 21 days (3 weeks), followed by a 7-day period (1 week) when no capsules are taken.
This cycle is repeated every 4 weeks.
Your doctor will regularly review you and you will normally keep taking Fotivda while it is working and you are not suffering from unacceptable side effects.
Reduced dose
If you experience severe side effects, your doctor may decide to interrupt treatment with Fotivda and/or reduce the dose to:
One Fotivda 890 micrograms capsule, taken once daily for 21 days (3 weeks), followed by a 7-day period (1 week) when no capsules are taken.
This cycle is repeated every 4 weeks.
Liver problems
If you have liver problems, your doctor may reduce the frequency of taking your dose to every other day (i.e., one 1340 micrograms capsule on alternate days).
Using Fotivda with food and drink
Fotivda should be taken with a glass of water and can be taken with or without food. Swallow the capsule whole. Do not chew, dissolve, or open the capsule before swallowing it.
If you take more Fotivda than you should
Tell your doctor immediately if you have taken more than the prescribed dose of 1 capsule per day.
Taking too much Fotivda may make side effects more likely or more intense, especially high blood pressure. Get medical help immediatelyif you experience confusion, changes in your mental status, or headaches. These are all symptoms of high blood pressure.
If you forget to take Fotivda
If you have missed a capsule, do nottake a capsule to replace it. Continue taking the next dose at the scheduled time.
Do nottake a double dose to make up for a missed capsule.
If you vomit after taking Fotivda, do nottake a capsule to replace it. Continue taking the next dose at the scheduled time.
If you stop taking Fotivda
Do not stop taking this medicine unless your doctor tells you to. If you stop taking the capsules, your disease may get worse.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
Severe Adverse Effects
Hypertensionis the most severe and very frequent adverse effect (see also section 2 "Warnings and Precautions").
Inform your doctor immediatelyif you think you have high blood pressure.Symptoms include severe headaches, blurred vision, shortness of breath, changes in your mental state, such as feeling anxious, confused, or disoriented.
Your doctor will check your blood pressure periodically during treatment with Fotivda. If you develop high blood pressure, your doctor may prescribe a medicine to treat hypertension, reduce your dose of Fotivda, or stop your treatment with Fotivda.
Other Adverse Effects
Very Common(may affect more than 1 in 10 people)
- Difficulty speaking
- Diarrhea
- Lack of appetite; weight loss
- Headache
- Difficulty breathing; shortness of breath during exercise; cough.
- Fatigue; unusual weakness; pain (oral, bone, in limbs, body sides, groin, tumor)
- Mouth inflammation; pain or mild discomfort in the mouth; feeling sick; pain, discomfort, and oppression in the stomach
- Palmoplantar syndrome with skin redness, swelling, numbness, and skin peeling on the palms and soles of the feet
- Back pain
- Fatigue and lack of energy
Common(may affect up to 1 in 10 people)
- Low thyroid gland activity, which can cause symptoms such as fatigue, lethargy, muscle weakness, slow heart rate, weight gain
- Insomnia
- Neurological damage, including numbness, tingling, sensitive skin, or numbness and weakness in arms and legs
- Vision problems, including blurred vision
- Rapid heartbeats; chest oppression; heart attack/reduced blood flow to the heart; blood clot in an artery (blood vessel)
- Blood clot in the lung. Symptoms include cough, chest pain, sudden difficulty breathing, or coughing up blood
- Blood clot in a deep vein, such as in the leg
- Very high blood pressure leading to a stroke; skin redness
- Nosebleeds; runny nose; nasal congestion
- Flatulence; acidity; difficulty and pain when swallowing; sore throat; swollen stomach; swollen and painful tongue; painful and/or bleeding gums
- Changes in taste or loss of taste
- Dizziness; ringing in the ears; dizziness and feeling that the environment is spinning (vertigo)
- Bleeding, e.g., in the brain, mouth, gums, lungs, stomach, intestinal ulcers, female genitals, anus, adrenal gland
- Blood in cough; vomiting blood
- Pallor and fatigue due to excessive bleeding
- Feeling sick; indigestion; constipation; dry mouth
- Itching of the skin; rash; body itching; skin peeling; dry skin; hair loss; skin redness, including hands and body; acne
- Fever; chest pain; swelling of feet and legs; chills and low body temperature
- Joint pain; muscle pain
- Increased amount of protein in the urine
- Abnormal blood test results for the liver, pancreas, kidneys, and thyroid
- Pancreatitis causing severe stomach pain that can radiate to the back
Uncommon(may affect up to 1 in 100 people)
- Pustular eruptions; fungal infections
- Easily bruising, bleeding into the skin
- Overactive thyroid gland (which can cause symptoms such as increased appetite, weight loss, heat intolerance, increased sweating, tremors, rapid heart rate); enlarged thyroid gland
- Increased red blood cell count
- Memory loss
- Transient reduction in blood flow to the brain
- Watery eyes
- Ear blockage
- Lack of blood flow through the heart's blood vessels
- Peptic ulcer in the small intestine
- Redness, swelling, and pain in the skin; skin blisters; excessive sweating; hives
- Muscle weakness
- Swelling or irritation of the mucous membranes
- Rapid and/or irregular heartbeat
- Heart failure. Symptoms include shortness of breath or swelling of the ankles. Swelling in the lungs due to fluid accumulation
Rare(may affect up to 1 in 1,000 people)
- Posterior reversible encephalopathy syndrome (PRES). Symptoms include headache, seizures, lack of energy, confusion, blindness, or other visual and neurological disturbances.
Frequency Not Known(cannot be estimated from the available data)
- Increased and weakened blood vessel wall or tear of the blood vessel wall (aneurysms and arterial dissections)
Reporting of Adverse Effects
If you experience adverse effects, consult your doctor, pharmacist, or nurse, even if they are adverse effects not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Fotivda
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and bottle after CAD. The expiration date is the last day of the month indicated.
Keep the bottle tightly closed to protect it from moisture.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Fotivda Contents
Fotivda 890 micrograms hard capsules
The active substance is tivozanib. Each hard capsule contains tivozanib hydrochloride monohydrate equivalent to 890 micrograms of tivozanib.
The other ingredients are:
- Capsule content: mannitol, magnesium stearate.
- Capsule shell: gelatin, titanium dioxide (E171), indigo carmine (E132), yellow iron oxide (E172).
- Yellow printing ink: shellac, propylene glycol, strong ammonia solution, titanium dioxide (E171), aluminum lake tartrazine (E102) (see section 2 "Fotivda contains tartrazine (E102)").
- Blue printing ink: shellac, propylene glycol, strong ammonia solution, aluminum lake indigo carmine (E132).
Fotivda 1340 micrograms hard capsules
The active substance is tivozanib. Each capsule contains tivozanib hydrochloride monohydrate equivalent to 1340 micrograms of tivozanib.
The other ingredients are:
- Capsule content: mannitol, magnesium stearate.
- Capsule shell: gelatin, titanium dioxide (E171), yellow iron oxide (E172).
- Blue printing ink: shellac, propylene glycol, strong ammonia solution, aluminum lake indigo carmine (E132).
Product Appearance and Package Contents
Fotivda 890 micrograms hard capsules have a dark blue opaque cap and a bright yellow opaque body, with "TIVZ" printed on the cap with yellow ink and "LD" on the body with dark blue ink.
Fotivda 1340 micrograms hard capsules have a bright yellow opaque cap and a bright yellow opaque body, with "TIVZ" printed on the cap with dark blue ink and "SD" on the body with dark blue ink.
Fotivda 890 micrograms and Fotivda 1340 micrograms are available as packs of 21 capsules in HDPE bottles with child-resistant closures.
Marketing Authorization Holder
Recordati Netherlands B.V.Beechavenue 54,
1119PW Schiphol-Rijk
Netherlands
Manufacturer
ALMAC PHARMA SERVICES (IRELAND) LIMITED
Finnabair Industrial Estate
Dundalk
Co. Louth
A91 P9KD
Ireland
Date of Last Revision of this Leaflet: 07/2023
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FOTIVDA 1340 micrograms hard capsulesDosage form: CAPSULE, 890 microgramsActive substance: tivozanibManufacturer: Recordati Netherlands B.V.Prescription requiredDosage form: TABLET, 1 mgActive substance: axitinibManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 3 mgActive substance: axitinibManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for FOTIVDA 1340 micrograms hard capsules
Discuss questions about FOTIVDA 1340 micrograms hard capsules, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






