
FLECTORMED 180 MG MEDICATED ADHESIVE PATCH

How to use FLECTORMED 180 MG MEDICATED ADHESIVE PATCH
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Flectormed 180mg medicated adhesive patch
diclofenac epolamine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Follow exactly the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or if it does not improve after 7 days.
Contents of the Package Leaflet
- What is Flectormed and what is it used for
- What you need to know before you start using Flectormed
- How to use Flectormed
- Possible side effects
- Storage of Flectormed
- Contents of the pack and further information
1. What is Flectormed and what is it used for
Indicated in adults and adolescents over 16 years.
Flectormed belongs to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs reduce pain and inflammation.
Flectormed is used for the symptomatic local and short-term treatment of minor painful disorders affecting the joints, muscles, tendons, and ligaments.
Children and adolescents under 16 years
This medicine is not recommended for use in children and adolescents under 16 years, as there is not enough data on the safety and efficacy of this medicine.
2. What you need to know before you start using Flectormed
Do not use Flectormed:
- if you are allergic to diclofenac epolamine or any of the other ingredients of this medicine (listed in section 6);
- if you are allergic to any pain relief medicine (analgesic) or anti-inflammatory medicine (NSAID);
- if you have asthma, breathing problems, skin rash, or nasal secretion after taking acetylsalicylic acid (aspirin) or other NSAIDs;
- if you have skin lesions, including infected or suppurating areas, eczema, burns, or wounds;
- if you are in your 6th month of pregnancy or later;
- if you currently have stomach ulcers (gastric or duodenal ulcers).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Flectormed if:
- you have breathing problems (allergic rhinitis, nasal polyps, asthma, chronic bronchial diseases);
- you have kidney, heart, or liver disease;
- you have had stomach or duodenal ulcers;
- you have had inflammatory bowel disease;
- you are prone to bleeding;
- you are in the first 6 months of pregnancy or breastfeeding.
Other important information
Always use the lowest effective dose of Flectormed for the shortest duration necessary to control the symptoms.
This medicine should be used with caution in elderly patients who may be more prone to side effects.
To minimize the frequency of side effects, it is recommended to use the lowest effective dose for the shortest possible time.
Do not use this medicine at the same time as another medicine that contains diclofenac or other non-steroidal anti-inflammatory drugs (NSAIDs).
Avoid exposure to direct sunlight or sunlamp for at least one day after removing the patch to reduce the risk of photosensitivity.
Be careful when using non-occlusive dressings, avoiding only occlusive dressings.
Stop using the medicated adhesive patch and inform your doctor or pharmacist if you experience skin rash when applying the patch or general hypersensitivity events (especially after prolonged treatment).
Children and adolescents
Do not use in children under 16 years.
Elderly
In this case, you should pay more attention while using the medicine to detect possible side effects.
People with liver or kidney problems
If you have liver or kidney problems, pay more attention while using the medicine to detect possible side effects.
Other medicines and Flectormed
If used correctly, the risk of interaction with other medicines is very low. However, inform your doctor or pharmacist if you are using or taking any other medicine, even if it was recently or if it will be in the future.
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. Flectormed should not be used if you are in your 6th month of pregnancy or later, as it may harm your fetus or cause problems during delivery.
Breastfeeding
During breastfeeding, do not apply to the breasts of nursing women or to large areas of skin or for a prolonged period.
Fertility
As with other NSAIDs, the use of diclofenac may affect fertility in women and is not recommended if you are planning to have a child. In women who may have difficulty conceiving or undergoing infertility treatment, the use of diclofenac should be avoided.
Driving and using machines
The influence of this medicine on the ability to drive and use machines is negligible.
This medicine contains
- 420 mg of propylene glycol, which may cause skin irritation.
- methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), and perfume DH Dalin, which contains amyl cinnamaldehyde, amyl cinnamaldehyde alcohol, benzyl alcohol, benzyl benzoate, benzyl salicylate, cinnamaldehyde, cinnamic alcohol, citronellol, d-limonene, eugenol, farnesol, geraniol, hexyl cinnamaldehyde, hydroxycitronellal, isoeugenol, linalool, methyl heptine carbonate, which may cause allergic reactions (possibly delayed).
3. How to use Flectormed
Follow exactly the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist.
Adults and adolescents over 16 years
Use one medicated adhesive patch per day for a maximum of 7 days. You should consult a doctor if you do not see improvement during the recommended treatment duration or if symptoms worsen.
This medicated adhesive patch should not be used in children or adolescents under 16 years, as there is not enough data on the safety and efficacy of this medicine.
Method of administration
Instructions for use for packs of 2, 5, and 10 medicated adhesive patches:
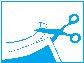
- Cut the top of the resealable pouch
containing the medicated adhesive patches along the dotted line.

- Remove a medicated adhesive patch and close the pouch
carefully by pressing the resealable closure.
- Remove the plastic backing that protects
the adhesive side of the medicated adhesive patch.

- Apply the medicated adhesive patch to
the skin, in the painful or inflamed area.
Instructions for use only for packs of 7 medicated adhesive patches:
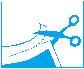
- Cut the top of the sealed pouch along
the dotted line and remove the medicated adhesive patch.
- Remove the plastic backing that protects
the adhesive side of the medicated adhesive patch.

- Apply the medicated adhesive patch to
the skin, in the painful or inflamed area.
If necessary, the patch can be kept in place using a non-occlusive dressing or the elastic mesh contained in the box, avoiding the use of occlusive dressings.
The medicated adhesive patch should only be applied to intact skin that is not damaged, avoiding application to open wounds or lesions. The patch should not be used during bathing or showering.
The medicated adhesive patch should be used whole, for the shortest possible treatment period, according to the instructions for use.
If you forget to use Flectormed
Do not use more than one medicated adhesive patch at the same time. If you need more information, contact your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects are rare and may be serious
If you experience any of the following signs of allergy, STOP using Flectormed and consult a doctor or pharmacist immediately:
- Swelling of lips, eyes, or tongue, wheezing, or asthma attack, which are signs of severe allergic reaction.
- Burning or stinging at the application site.
Generally, side effects are related to the application site.
Common side effects(affect 1 to 10 users in 100)
Skin reactions: skin rash, dermatitis, redness, and inflammation of the skin, itching.
Other side effects
Uncommon, rare, and very rare: skin rash with blistering or vesicles, dry skin, allergic reaction also after skin exposure to sunlight or sunlamp (photosensitivity), hives.
If the medicated adhesive patches are used correctly, the risk of side effects is very low, whereas if they are used for prolonged treatment periods or together with other medicines containing diclofenac, especially orally, the risk of generalized side effects cannot be excluded.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Flectormed
Keep this medicine out of the sight and reach of children.
Store below 30°C.
Only for packs of 2, 5, and 10 medicated adhesive patches: do not use after 3 months from the first opening of the pouch. After removing each medicated adhesive patch, make sure the pouch is resealed.
Do not use this medicine after the expiry date stated on the pouch and carton after EXP.
Do not throw away unused medicated adhesive patches, they should be disposed of according to local regulations. Used medicated adhesive patches should not be thrown into the toilet or deposited into liquid waste disposal systems.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicines to the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Flectormed
The active substance is diclofenac epolamine. Each medicated adhesive patch contains a total of 180 mg of diclofenac epolamine, equivalent to 140 mg of diclofenac sodium (1% w/w).
The other ingredients are gelatin, povidone (K90), sodium heparin, liquid sorbitol, kaolin, titanium dioxide (E171), propylene glycol, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), disodium edetate (E385), tartaric acid, aluminum glycinate, sodium carmellose, sodium polyacrylate, 1,3-butylene glycol, polysorbate 80, fragrance (which contains amyl cinnamaldehyde, amyl cinnamaldehyde alcohol, benzyl alcohol, benzyl benzoate, benzyl salicylate, cinnamaldehyde, cinnamic alcohol, citronellol, d-limonene, eugenol, farnesol, geraniol, hexyl cinnamaldehyde, hydroxycitronellal, isoeugenol, linalool, methyl heptine carbonate), purified water, and non-woven polyester backing.
Appearance and packaging
Each medicated adhesive patch consists of a paste of a color between white and pale yellow impregnated into a patch with a removable plastic backing that protects the adhesive layer.
The carton contains 2, 5, or 10 medicated adhesive patches in 1 or 2 resealable pouches, while the carton of 7 medicated adhesive patches contains 7 sealed pouches with one medicated adhesive patch each.
A tubular mesh is included.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi, Italy
Manufacturer
Altergon Italia srl
Zona Industriale ASI
83040 Morra De Sanctis (AV), Italy
You can request more information about this medicine from the local representative of the marketing authorization holder:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Spain)
This medicine is authorized in the following European Economic Area member states with the following names:
Belgium Flalgo/Flectoflex
Czech Republic Flalgo
Hungary Flectorin
Italy Calminemed
Slovakia Flectopar
Spain Flectormed
Date of last revision of this leaflet:October 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLECTORMED 180 MG MEDICATED ADHESIVE PATCHDosage form: GEL, 10 mg/gActive substance: diclofenacManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: GEL, 23.2 mg/gActive substance: diclofenacManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: TOPICAL SOLUTION, 39.2 mg/mlActive substance: diclofenacManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for FLECTORMED 180 MG MEDICATED ADHESIVE PATCH
Discuss questions about FLECTORMED 180 MG MEDICATED ADHESIVE PATCH, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






