FILSUVEZ GEL

How to use FILSUVEZ GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Filsuvez Gel
Birch Bark Extract
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Filsuvez and what is it used for
- What you need to know before you use Filsuvez
- How to use Filsuvez
- Possible side effects
- Storage of Filsuvez
- Contents of the pack and other information
1. What is Filsuvez and what is it used for
Filsuvez gel is a herbal medicine that contains dry extract of birch bark.
It is used for the treatment of wounds in adults and children (from 6 months of age) who suffer from a type of disease called "epidermolysis bullosa" (EB), specifically "dystrophic" (DEB) or "junctural" (JEB). This is a condition where the outer layer of the skin separates from the inner layer, making the skin very fragile and causing wounds to appear.
2. What you need to know before you use Filsuvez
Do not use Filsuvez
- if you are allergic to birch bark or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Filsuvez.
If you have an allergic reaction, stop using Filsuvez immediatelyand go to your doctor or nurse. Signs of an allergic reaction include:
- itching, swelling, and redness of the skin that is more severe in the area where the medicine has been applied.
Wound infection is a serious complicationthat can occur during the healing process. Possible signs of wound infection are:
- yellow or greenish liquid (pus) coming out of the wound,
- red, hot, swollen, or increasingly painful skin around the wound.
If you have a wound infection, you may need to stop using Filsuvezand another treatment may be necessary. Your doctor or nurse will tell you if Filsuvez treatment can be restarted once the infection has cleared up.
People with EB are more likely to develop a type of skin cancer called "squamous cell carcinoma" (SCC). If you are diagnosed with skin cancer while using Filsuvez, you should talk to your doctor or nurse and stop using Filsuvezon that part of the skin.
Filsuvez does notcontain birch pollen, so it can be used in people with a birch pollen allergy.
Avoid getting Filsuvez in your eyes. If this happens, rinse your eyes well with clean water. Contact your doctor or nurse if the discomfort continues.
Children
Do not give this medicine to children under 6 months of age.
Other medicines and Filsuvez
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
There is no information on how Filsuvez might interact with other medicines applied to the skin, taken by mouth, or injected. Do not apply other products to the wound area at the same time as applying Filsuvez. If you need to use more than one product, talk to your doctor or nurse.
Pregnancy, breastfeeding, and fertility
No studies have been done on the effects of Filsuvez in pregnant women, but since the absorption of this medicine into the body is extremely low, the risk to the fetus is negligible. Filsuvez can be used during pregnancy.
It is not known if Filsuvez passes into breast milk, but since the absorption of this medicine into the body is extremely low, the risk to the fetus is negligible. Filsuvez can be used during breastfeeding, unless the breast area is being treated.
Since the absorption of this medicine into the body is extremely low, it is not expected to affect your fertility.
Driving and using machines
Your ability to drive and use machines will not be affected by this medicine.
3. How to use Filsuvez
Follow the instructions for administration of this medicine exactly as told by your doctor, pharmacist, or nurse. If you are unsure, talk to your doctor, pharmacist, or nurse again.
Methods of administration
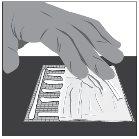
- Clean the wound before applying Filsuvez.
- You can apply Filsuvez in two ways:
- Apply directly to the wound
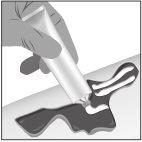
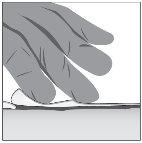
- Apply a thick layer (about 1 mm thick) of Filsuvez to the wound (step 1).
- Spread the gel generously and cover the entire wound area with a clean hand or glove (step 2). Do notrub the gel.
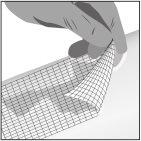
- Cover with a non-adhesive sterile dressing (step 3).
Step 1: Apply | Step 2: Spread | Step 3: Cover |
|
|
|
Or
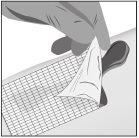
- Apply to a non-adhesive sterile dressing
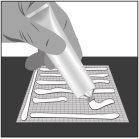
- Apply a thick layer (about 1 mm thick) of Filsuvez to the wound dressing (step 1).
- Spread generous amounts of gel to the area that will be in direct contact with the wound with a clean hand or glove (step 2).
- Cover the wound with the dressing (step 3).
Step 1: Apply | Step 2: Spread | Step 3: Cover |
|
|
|
- Reapply the gel every time the dressing is changed, until the wound is healed.
- Filsuvez is not intendedfor internal use. Avoid contact with the eyes, mouth, or nostrils. In case of accidental contact, rinse immediately with clean water.
- This tube of sterile gel is intended for single use. Once opened, the gel must be used immediately and the tube must be discarded, even if some gel remains. A new tube should be used for each dressing change.
Duration of treatment
Your doctor, pharmacist, or nurse will tell you how long you should use the gel. If your symptoms continue or worsen after use, or if complications occur in the wound, talk to your doctor, pharmacist, or nurse.
If you use more Filsuvez than you should
Filsuvez is applied to the skin and absorption into the body is extremely low. This makes overdose very unlikely, even if applied to large areas of skin and for a long period of time.
If you forget to use Filsuvez
Apply Filsuvez at the next scheduled dressing change, continuing with your usual routine.
If you stop using Filsuvez
Filsuvez should be used as directed by your doctor, pharmacist, or nurse. Do not stop using itwithout talking to your doctor, pharmacist, or nurse.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Tell your doctor, pharmacist, or nurse immediately if you notice any of the following side effects, including those listed below.
Very common(may affect more than 1 in 10 people)
- wound complications (e.g., increased wound size, wound reopening, and pain in the wound)
Common(may affect up to 1 in 10 people)
- wound infection
- allergic reaction (hypersensitivity)
- itching of the skin
- pain and itching at the application site
- wound healing complications
Uncommon(may affect up to 1 in 100 people)
- wound secretion
- skin irritation (dermatitis)
- itchy rash
- purple-colored rash
- pain
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Filsuvez
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and tube after "EXP". The expiry date is the last day of the month shown.
Store below 30°C.
This tube of sterile gel is intended for single use. Once opened, the gel must be used immediately and the tube must be discarded, even if some gel remains. A new tube should be used for each dressing change.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Filsuvez
The active substance is dry extract of birch bark.
1 g of gel contains: 100 mg of extract (as refined dry extract) from Betula pendulaRoth, Betula pubescensEhrh., as well as hybrids of both species, bark (equivalent to 0.5-1.0 g of birch bark), including 84-95 mg of triterpenes calculated as the sum of betulin, betulinic acid, erythrodiol, lupeol, and oleanolic acid. Extraction solvent: n-heptane.
The other ingredient is refined sunflower oil.
Appearance of the product and pack contents
Filsuvez is a colorless to slightly yellowish, opalescent, and non-aqueous gel.
Filsuvez gel is available in white, foldable aluminum tubes. The tubes are closed with a tamper-evident aluminum membrane and are equipped with a white polypropylene screw cap.
The tube is packaged in a cardboard box.
Pack sizes:
1 tube, 10 tubes, and 30 tubes of 23.4 g gel.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Chiesi Farmaceutici S.p.A.
Via Palermo 26/A
43122 Parma
Italy
Manufacturer
Amryt GmbH
Streiflingsweg 11
75223 Niefern-Öschelbronn
Germany
You can request more information about this medicine from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Chiesi sa/nv Tel: + 32 (0)2 788 42 00 | Lietuva ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +370 661 663 99 |
| Luxembourg/Luxemburg Chiesi sa/nv Tel: + 32 (0)2 788 42 00 |
Ceská republika ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +420 724 321 774 | Magyarország ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +36 20 399 4269 |
Danmark Chiesi Pharma AB Tlf: + 46 8 753 35 20 | Malta Amryt Pharmaceuticals DAC Tel: +44 1604 549952 |
Deutschland Chiesi GmbH Tel: + 49 40 89724-0 | Nederland Chiesi Pharmaceuticals B.V. Tel: + 31 88 501 64 00 |
Eesti ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +370 661 663 99 | Norge Chiesi Pharma AB Tlf: + 46 8 753 35 20 |
Ελλάδα Amryt Pharmaceuticals DAC Τηλ: +800 44 474447 Τηλ: +44 1604 549952 | Österreich Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
España Chiesi España, S.A.U. Tel: + 34 93 494 8000 | Polska ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +48 502 188 023 |
France Chiesi S.A.S. Tél: + 33 1 47688899 | Portugal Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Hrvatska ExCEEd Orphan Distribution d.o.o. Savska cesta 32, Zagreb, 100 00 Croatia Tel: +385 99 320 0330 | România ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +40 744 366 015 |
Ireland Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Slovenija ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +386 30 210 050 |
Ísland Chiesi Pharma AB Sími: +46 8 753 35 20 | Slovenská republika ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +420 608 076 274 |
Italia Chiesi Italia S.p.A. Tel: + 39 0521 2791 | Suomi/Finland Chiesi Pharma AB Puh/Tel: +46 8 753 35 20 |
Κύπρος Amryt Pharmaceuticals DAC Τηλ: +800 44 474447 Τηλ: +44 1604 549952 | Sverige Chiesi Pharma AB Tel: +46 8 753 35 20 |
Latvija ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +370 661 663 99 |
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (https://www.aemps.gob.es/). There are also links to other websites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FILSUVEZ GELDosage form: OINTMENT, 10 mg/gActive substance: Centella asiatica herba, incl. combinationsManufacturer: Almirall S.A.Prescription not requiredDosage form: TOPICAL SOLID, 2 g Centella asiatica extract / 100 gActive substance: Centella asiatica herba, incl. combinationsManufacturer: Almirall S.A.Prescription not requiredDosage form: OINTMENT, 2 g / 15 gActive substance: Other cicatrizantsManufacturer: Alfasigma Espana S.L.Prescription not required
Online doctors for FILSUVEZ GEL
Discuss questions about FILSUVEZ GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions