FASENRA 30 mg Injectable Solution in Pre-filled Syringe

How to use FASENRA 30 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Fasenra 30mg solution for injection in pre-filled syringe
benralizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fasenra and what is it used for
- What you need to know before you use Fasenra
- How to use Fasenra
- Possible side effects
- Storage of Fasenra
- Contents of the pack and other information
1. What is Fasenra and what is it used for
Whatis Fasenra
Fasenra contains the active substance benralizumab, which is a monoclonal antibody, i.e. a type of protein that recognizes and binds to a specific substance in the body. The target of benralizumab is a protein called interleukin-5 receptor, which is found particularly in a type of white blood cell called an eosinophil.
What is Fasenra used for
Asthma
Fasenra is used to treat severe eosinophilic asthmain adults. Eosinophilic asthma is a type of asthma where patients have too many eosinophils in their blood or lungs.
Fasenra is used in addition to other medicines for asthma (high doses of ‘inhaled corticosteroids’ plus other anti-asthma medicines) when the disease is not well controlled with these other medicines alone.
Eosinophilic Granulomatosis with Polyangiitis (EGPA)
Fasenra is used to treat EGPA in adults. EGPA is a disease where people have too many eosinophils in their blood and tissues and also have some form of vasculitis. This means there is inflammation of the blood vessels. This disease most commonly affects the lungs and the sinuses, but often affects other organs such as the skin, heart, and kidneys.
How Fasenra works
Eosinophils are white blood cells involved in the inflammation of asthma and EGPA. By binding to eosinophils, Fasenra helps to reduce their frequency and inflammation.
Benefits of using Fasenra
Asthma
Fasenra may reduce the frequency of asthma attacks you are experiencing, helping you to breathe better and reducing your asthma symptoms. If you are using medicines called ‘oral corticosteroids’, using Fasenra may also allow you to reduce the daily dose or stop treatment with oral corticosteroids you need to control your asthma.
EGPA
Fasenra may reduce the symptoms and prevent flare-ups of EGPA. This medicine may also allow you to reduce the daily dose of oral corticosteroids you need to control your symptoms.
2. What you need to know before you use Fasenra
Do not use Fasenra:
- If you are allergicto benralizumab or any of the other ingredients of this medicine (listed in section 6). Talk to your doctor, nurse or pharmacistif you think this applies to you.
Warnings and precautions
Talk to your doctor, nurse or pharmacist before using Fasenra:
Also, talk to your doctor, pharmacist or nurse if you are receiving Fasenra:
Fasenra is not a rescue medicine.Do not use it to treat a sudden asthma attack.
Be aware of the signs of serious allergic reactions
Fasenra may potentially cause serious allergic reactions. You should be aware of the signs of these reactions (such as hives, skin rash, difficulty breathing, fainting, discomfort, feeling dizzy and/or swelling of the face, tongue or mouth) while you are receiving Fasenra.
It is important that you talk to your doctor about how to recognize the early symptoms of serious allergic reactions and how to manage them if they occur.
In order to improve the traceability of biological medicinal products, the name and batch number of the product should be clearly recorded in the patient file each time the product is administered, and this information should be reported when reporting any adverse reactions.
Other medicines for asthma or EGPA
Do not suddenly stopor change the dose of your other medicines for your disease when you start Fasenra.
If your response to treatment allows, your doctor may try to reduce the dose of some of these medicines, especially those called "corticosteroids". This should be done gradually and under the direct supervision of your doctor.
Tell your doctorif you are using, have recently used, or might use any other medicines before using Fasenra.
Children and adolescents
Do not give this medicine to children under 18 years of age because the safety and benefits of this medicine in this population are not known.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctorfor advice before using this medicine.
Do not use Fasenra if you are pregnant unless your doctor tells you to. It is not known if Fasenra can affect the fetus.
It is not known if the components of Fasenra can pass into breast milk. Talk to your doctorif you are breastfeeding or plan to breastfeed.
Driving and using machines
Fasenra is unlikely to affect your ability to drive or use machines.
Fasenra contains polysorbate 20
This medicine contains 0.06 mg of polysorbate 20 (of vegetable origin) in each 30 mg pre-filled syringe. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergy.
3. How to use Fasenra
Always use this medicine exactly as your doctor has told you. Check with your doctor, nurse or pharmacist if you are not sure.
Asthma
The recommended doseis one 30 mg injection. The first three injections are given every 4 weeks. After that, the injections are given every 8 weeks.
EGPA
The recommended doseis one 30 mg injection every 4 weeks.
Fasenra is given by injection just under the skin (subcutaneously). You and your doctor or nurse should decide if you should inject Fasenra yourself. You should not inject Fasenra yourself if you have not received Fasenra before, or if you have had a previous allergic reaction with Fasenra.
You or your caregiver should receive training on the correct way to inject Fasenra. Read the ‘Instructions for Use’ for the pre-filled syringe carefully before using Fasenra.
If youmiss a doseof Fasenra
If you have missed a dose of Fasenra, talk to your doctor, pharmacist, or nurse as soon as possible.
If youstop treatment with Fasenra
Do not stop treatment with Fasenra unless your doctor advises you to. Stopping or interrupting treatment with Fasenra may cause your symptoms to come back and asthma exacerbations.
If your asthma symptoms worsen while you are receiving Fasenra injections, talk to your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious allergic reactions
See a doctor immediatelyif you think you may be having an allergic reaction. These reactions can occur hours or days after the injection.
Frequency not known(cannot be estimated from the available data):
- anaphylaxis
Common symptoms include:
o swelling of the face, tongue or mouth
o breathing problems
o fainting, dizziness, lightheadedness (due to a drop in blood pressure)
Common(may affect up to 1 in 10 people)
- hypersensitivity reactions (hives, skin rash)
Other side effects
Common(may affect up to 1 in 10 people)
- headache
- pharyngitis (sore throat)
- fever (high temperature)
- injection site reaction (e.g. pain, redness, itching, swelling near the injection site)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fasenra
Keep this medicine out of the sight and reach of children.
Fasenra is for single use only.
Do not use this medicine after the expiry date which is stated on the label and carton after ‘EXP/CAD’. The expiry date is the last day of the month shown.
Store in the original package to protect from light.
Store in a refrigerator (2°C to 8°C).
The pre-filled syringe can be stored at room temperature (up to 25°C) for a maximum of 14 days. After removal from the refrigerator, Fasenra should be used within 14 days or discarded, and the discard date should be written on the carton.
Do not shake, freeze, or expose to heat.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Compositionof Fasenra
The active substance is benralizumab. A 1 ml pre-filled syringe of solution contains 30 mg of benralizumab.
The other components are histidine, histidine hydrochloride monohydrate, trehalose dihydrate, polysorbate 20 (E 432), and water for injectable preparations.
Appearance and Container Contents of the Product
Fasenra is a solution in a transparent glass syringe. Its color varies between colorless and yellow. It may contain particles.
Fasenra is available in a pack containing 1 pre-filled syringe.
Marketing Authorisation Holder
AstraZeneca AB
SE-151 85 Södertälje
Sweden
Manufacturer
AstraZeneca AB
Gärtunavägen
SE-152 57 Södertälje
Sweden
MedImmune UK Ltd
6 Renaissance Way
Liverpool, L24 9JW
United Kingdom
AstraZeneca Nijmegen B.V., Nijmegen
Lagelandseweg 78
Nijmegen, 6545CG
Netherlands
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
Belgium/Belgique/Belgien AstraZeneca S.A./N.V. Tel: +32 2 370 48 11 | Lithuania UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg AstraZeneca S.A./N.V. Tél/Tel: +32 2 370 48 11 |
Czech Republic AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Hungary AstraZeneca Kft. Tel.: +36 1 883 6500 |
Denmark AstraZeneca A/S Tlf.: +45 43 66 64 62 | Malta Associated Drug Co. Ltd Tel: +356 2277 8000 |
Germany AstraZeneca GmbH Tel: +49 40 809034100 | Netherlands AstraZeneca BV Tel: +31 85 808 9900 |
Estonia AstraZeneca Tel: +372 6549 600 | Norway AstraZeneca AS Tlf: +47 21 00 64 00 |
Greece AstraZeneca A.E. Τηλ: +30 210 6871500 | Austria AstraZeneca Österreich GmbH Tel: +43 1 711 31 0 |
Spain AstraZeneca Farmacéutica Spain, S.A. Tel: +34 91 301 91 00 | Poland AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
France AstraZeneca Tél: +33 1 41 29 40 00 | Portugal AstraZeneca Produtos Farmacêuticos, Lda. Tel: +351 21 434 61 00 |
Croatia AstraZeneca d.o.o. Tel: +385 1 4628 000 | Romania AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland AstraZeneca Pharmaceuticals (Ireland) DAC Tel: +353 1609 7100 | Slovenia AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Iceland Vistor hf. Sími: +354 535 7000 | Slovak Republic AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italy AstraZeneca S.p.A. Tel: +39 02 00704500 | Finland AstraZeneca Oy Puh/Tel: +358 10 23 010 |
Cyprus Αλκήτωρ Φαρμακευτική Λτδ Τηλ: +357 22490305 | Sweden AstraZeneca AB Tel: +46 8 553 26 000 |
Latvia SIA AstraZeneca Latvija Tel: +371 67377100 | United Kingdom (Northern Ireland) AstraZeneca UK Ltd Tel: +44 1582 836 836 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
<---------------------------------------------------------------------------------------------------------------->
Instructions for Use
Fasenra 30 mg solution for injection in pre-filled syringe
benralizumab
For subcutaneous injection
Single-use pre-filled syringe
Before starting to use Fasenra pre-filled syringe, a healthcare professional should teach you or your caregiver how to use it correctly.
Read these “Instructions for Use” before starting to use Fasenra pre-filled syringe and each time you have to perform a new injection.There may be new information. This information does not replace consultation with your healthcare professional regarding your disease or treatment.
If you or your caregiver has any questions, consult your healthcare professional.
Important Information
Store Fasenra in a refrigerator between 2°C and 8°C in its carton until you are ready to use it.Fasenra may be stored at room temperature up to 25 °C for a maximum of 14 days. After removal from the refrigerator, Fasenra must be used within 14 days or discarded.
Do not useyour Fasenra pre-filled syringe if:
| Do not:
|
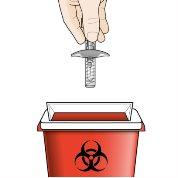
If any of the above occurs, discard the syringe in a puncture-resistant container for sharp objects and use a new pre-filled syringe.
Each Fasenra pre-filled syringe contains 1 dose of Fasenra, which is for single use only.
Keep Fasenra and all medicines out of the sight and reach of children.
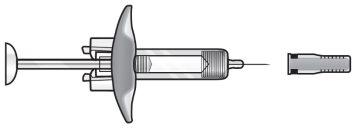
Your Fasenra pre-filled syringe
Do not removethe needle cap until you have reached Step 6 and are ready to inject Fasenra.
Do not touchthe needle shield activation clips to avoid activating the safety device (needle shield) too early.
Needle shield activation clips | Syringe body | Expiry date label | Needle cap | |||
Plunger head |
| |||||
Plunger | Finger grip | Viewing window | Needle |
Step 1 – Gather materials
- 1 Fasenra pre-filled syringe from the refrigerator
- 1 alcohol swab
- 1 cotton ball or gauze
- 1 puncture-resistant container for sharp objects.
(See Step 9 – Dispose of the pre-filled syringe)
|
|
|
|
Pre-filled syringe | Alcohol swab | Cotton ball or gauze | Puncture-resistant container for sharp objects |
Step 2 – Prepare your pre-filled syringe | |
Check the expiry date (EXP).Do not use it if the expiry date has passed. Before administration, allow the pre-filled syringe to reach room temperature (20°C to 25°C) by leaving the carton outside the refrigerator for approximately 30minutes. Do notheat the pre-filled syringe in any other way. For example, do not heat it in a microwave or with hot water, or place it near heat sources. Use Fasenra within 14 days of removal from the refrigerator. |
|
Step 3 – Check the liquid | |
Hold the syringe body(notthe plunger) to remove the syringe. Look at the liquid through the viewing window.The liquid should be clear and colorless to yellowish. It may contain small white particles. Do notinject Fasenra if the liquid is cloudy, discolored, or contains large particles. You may see a small air bubble in the liquid. This is normal. |
|
Step 4 – Choose the injection site | |
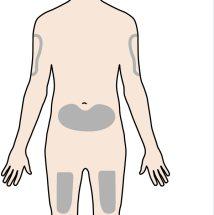
The recommended injection site is the front of the thigh. You may also use the lower abdomen. Do notinject:
A caregiver may inject it into the upper arm, thigh, or abdomen. Do notattempt to inject it yourself into the upper arm. For each injection, choose a different site, at least 3 cm away from the site of the previous injection. |
|
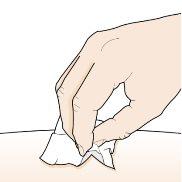
Step 5 – Clean the injection site | |
Wash your hands well with soap and water. Clean the injection site with an alcohol swab in a circular motion. Let it air dry. Do nottouch the cleaned area before injection. Do notfan or blow on the cleaned area. |
|
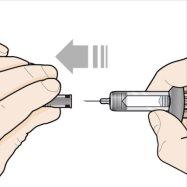
Step 6 – Remove the needle cap | |
Hold the syringe with one hand, and carefully pull the needle cap with the other hand. Do nothold the plunger or plunger head while removing the needle cap. Set the needle cap aside to discard later. You may see a drop of liquid at the end of the needle. This is normal. Do notuse the syringe if it has been dropped without the needle cap in place or if the needle is damaged or dirty. Do nottouch the needle or let it touch any surface. Proceed directly to the next steps without pause. |
|
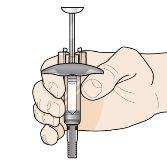
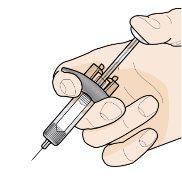
Step 7 – Inject Fasenra | |
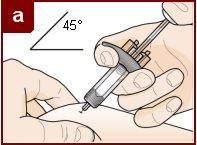
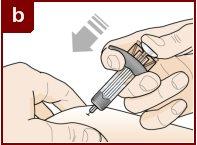
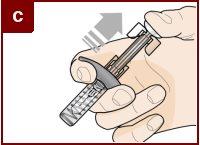
Hold the pre-filled syringe with one hand, as shown in the figure. Use the other hand to gently pinch and hold the skin area where you want to inject. This creates a firmer surface. Do notpress the plunger until the needle is fully inserted into the skin. Do notpull back the plunger at any time. Inject Fasenra following the steps in figures a, b, and c. |
|
|
|
|
Make a quick, dart-like motion to insert the needle into the pinched skin. Insert the needle at a 45-degree angle. | Use your thumb to push the plunger head. Continue pushing the plunger until it reaches the bottom. This ensures that all the medication is injected. | Keep your thumb pushing the plunger head while withdrawing the needle from the skin. Stop pressing the plunger slightly until the needle shield activation clips cover the needle. |
Step 8 – Check the injection site | |
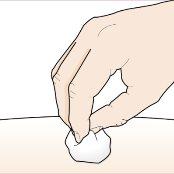
You may see a small amount of blood or liquid at the injection site. This is normal. Gently press on the skin with a cotton ball or gauze until bleeding stops. Do notrub the injection site. If necessary, cover the injection site with a small bandage. |
|
Step 9 – Dispose of the used pre-filled syringe | |
Do notthrow the pre-filled syringe in your household trash. Do notrecap the pre-filled syringe. Discard the needle cap and any other used materials in your household trash. |
|
Disposal Guide Dispose of the container as a whole according to the instructions of your healthcare professional or pharmacist. Do notrecycle your puncture-resistant container for sharp objects. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FASENRA 30 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 30 mgActive substance: benralizumabManufacturer: Astrazeneca AbPrescription requiredDosage form: INJECTABLE PERFUSION, 100 mg injectable 10 mlActive substance: reslizumabManufacturer: Teva B.V.Prescription requiredDosage form: TABLET, 250 µgActive substance: roflumilastManufacturer: Astrazeneca AbPrescription required
Online doctors for FASENRA 30 mg Injectable Solution in Pre-filled Syringe
Discuss questions about FASENRA 30 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions