EYLEA 114.3 mg/mL Injectable Solution

How to use EYLEA 114.3 mg/mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Eylea 114.3 mg/ml Solution for Injection
aflibercept
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Eylea and what is it used for
- What you need to know before you are given Eylea
- How Eylea will be given
- Possible side effects
- Storage of Eylea
- Contents of the pack and other information
1. What is Eylea and what is it used for
What is Eylea
Eylea contains the active substance aflibercept. It belongs to a group of medicines called anti-angiogenic agents.
Your doctor will inject Eylea into your eye to treat certain eye disorders in adult patients called:
- age-related macular degeneration (wet AMD)
- vision impairment due to diabetic macular edema (DME).
These disorders affect the macula. The macula is the central part of the light-sensitive membrane at the back of the eye. It is responsible for clear vision.
Wet AMD occurs when abnormal blood vessels form and grow under the macula. These abnormal blood vessels can leak fluid or blood into the eye. Leaking blood vessels that cause swelling of the macula lead to DME. Both disorders can affect your vision.
How Eylea works
Eylea stops the growth of new abnormal blood vessels in the eye. Eylea may help to stabilize and, in many cases, improve vision.
2. What you need to know before you are given Eylea
You will not be given Eylea if
- you are allergic to aflibercept or any of the other ingredients of this medicine (listed in section 6)
- you have an eye infection or infection around the eye
- you have eye pain or redness (severe eye inflammation).
Warnings and precautions
Talk to your doctor before you are givenEylea if:
- you have glaucoma, an eye disease caused by high pressure in the eye
- you have a history of flashes of light or floaters and if their size or number suddenly increases
- you have had eye surgery in the last 4 weeks or have scheduled eye surgery in the next 4 weeks.
Tell your doctor immediately ifyou experience:
- eye redness
- eye pain
- increased eye discomfort
- blurred or decreased vision
- increased sensitivity to light
These may be symptoms of inflammation or infection and your doctor may stop treatment with Eylea.
In addition, it is important that you know that:
- the safety and efficacy of Eylea when given in both eyes at the same time have not been studied and such use may increase the risk of side effects.
- Eylea injections may cause an increase in eye pressure in some patients within 60 minutes after injection. Your doctor will monitor you after each injection.
- your doctor will check for other risk factors that may increase the likelihood of a tear or detachment of the layers at the back of the eye. In such cases, your doctor will give you Eylea with caution.
- women of childbearing age must use effective contraceptive methods during treatment and for at least 4 months after the last Eylea injection.
The use of substances similar to those in Eylea is potentially related to the risk of blockage of blood vessels by blood clots, which can lead to a heart attack or stroke. In theory, this could also occur after an Eylea injection into the eye. If you have had a stroke, a transient ischemic attack, or a heart attack in the last 6 months, your doctor will give you Eylea with caution.
Children and adolescents
Eylea has not been studied in children and adolescents under 18 years of age, as the indicated diseases mainly occur in adults. Therefore, its use in this age group is not recommended.
Other medicines and Eylea
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- Women who may become pregnant must use effective birth control methods during treatment and for at least 4 months after the last Eylea injection.
- There is limited experience with the use of Eylea in pregnant women. Women should not receive Eylea during pregnancy unless the potential benefit to the woman outweighs the potential risk to the fetus.
- Small amounts of Eylea may pass into breast milk. The effect on newborns/infants is unknown. The use of Eylea is not recommended during breastfeeding.
Therefore, if you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before receiving this medicine.
Driving and using machines
After Eylea injection, you may experience temporary vision problems. Do not drive or use machines while these problems last.
Eylea contains polysorbate 20
This medicine contains 0.021 mg of polysorbate 20 in each 0.07 ml dose, equivalent to 0.3 mg/ml.
Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How Eylea will be given
The recommended dose is 8 mg of aflibercept per injection.
- You will receive 1 injection per month for the first 3 months.
- After this, you may receive injections every 6 months. Your doctor will decide on the frequency based on the condition of your eye.
- If your doctor changes your treatment to Eylea 114.3 mg/ml, your doctor will decide on the frequency after the first injection.
Method of administration
Your doctor will inject Eylea into the inside of your eye (intravitreal injection).
Before the injection, your doctor will use an eye wash disinfectant to carefully clean your eye to prevent infection. Your doctor will give you eye drops (local anesthetic) to numb the eye in order to reduce or prevent pain from the injection.
If you miss an Eylea injection
Make a new appointment with your doctor as soon as possible.
Before stopping treatment with Eylea
Talk to your doctor before stopping treatment. Stopping treatment may increase the risk of vision loss and your vision may worsen.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects of Eylea injection are due to the medicine itself or the injection procedure and mostly affect the eye.
Some side effects can be serious
Contact your doctor immediately if you experience any of the following:
- common side effect, which may affect up to 1 in 10 people
- clouding of the lens (cataract)
- bleeding in the back of the eye (retinal hemorrhage)
- increased pressure in the eye
- bleeding in the eye (vitreous hemorrhage)
- uncommon side effect, which may affect up to 1 in 100 people
- certain types of clouding of the lens (subcapsular/nuclear cataract)
- detachment, tear, or hemorrhage of the light-sensitive membrane in the back of the eye, which can cause flashes of light with floaters that sometimes progress to vision loss (retinal tear or detachment)
Other possible side effects
Common(may affect up to 1 in 10 people):
- allergic reactions
- floaters in the vision (particles in the vitreous humor)
- detachment of the gel-like substance in the eye (vitreous detachment)
- decreased visual acuity
- eye pain
- bleeding in the eye (conjunctival hemorrhage)
- damage to the clear layer on the front of the eye (punctate keratitis, corneal abrasion)
Uncommon(may affect up to 1 in 100 people):
- detachment or tear of one of the layers in the back of the eye, which can cause flashes of light with floaters that sometimes progress to vision loss (retinal pigment epithelial tear or detachment)
- inflammation of the iris, other parts of the eye, or the gel-like substance in the eye (uveitis, iritis, iridocyclitis, vitritis)
- certain types of clouding of the lens (cortical cataract)
- damage to the surface of the eye (corneal erosion)
- blurred vision
- pain at the injection site
- sensation of having something in the eye
- increased tear production
- bleeding at the injection site
- redness of the eye
- swelling of the eyelid
- redness of the eye (ocular hyperemia)
- irritation at the injection site
Rare(may affect up to 1 in 1,000 people):
- swelling of the clear layer on the front of the eye (corneal edema)
- clouding of the lens (lenticular opacity)
- degeneration of the light-sensitive membrane in the back of the eye (retinal degeneration)
- irritation of the eyelid
Frequency not known(cannot be estimated from the available data):
- inflammation of the white part of the eye associated with redness and pain (scleritis)
In addition to the above, the following side effects may occur:
- abnormal sensation in the eye
- damage to the surface of the clear layer on the front of the eye (corneal epithelial defect)
- inflammation of other parts of the eye (cells in the anterior chamber)
- severe inflammation or infection inside the eye (endophthalmitis)
- blindness
- clouding of the lens due to injury (traumatic cataract)
- pus in the eye (hypopyon)
- severe allergic reactions
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Eylea
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and label after “EXP”. The expiry date refers to the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C). Do not freeze.
- The unopened vial can be stored below 25°C for a maximum of 24 hours.
- Keep the vial in the outer packaging to protect it from light.
6. Container contents and additional information
Eylea composition
- The active substance is aflibercept. 1 ml of solution contains 114.3 mg of aflibercept. Each vial contains 0.263 ml. This provides a usable amount to administer a single dose of 0.07 ml containing 8 mg of aflibercept.
- The other components are: sucrose, arginine hydrochloride, histidine hydrochloride monohydrate, histidine, polysorbate 20, water for injectable preparations.
See "Eylea contains polysorbate 20" in section 2 for more information.
Appearance of Eylea and container contents
Eylea is an injectable solution (injectable). The solution is colorless to pale yellow.
Container: 1 vial + 1 filter needle.
Marketing authorization holder
Bayer AG
51368 Leverkusen
Germany
Manufacturer
Bayer AG
Müllerstraße 178
13353 Berlin
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Bayer SA-NV Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel: +37 05 23 36 868 |
| Luxembourg/Luxemburg Bayer SA-NV Tel: +32-(0)2-535 63 11 |
Ceská republika Bayer s.r.o. Tel: +420 266 101 111 | Magyarország Bayer Hungária KFT Tel: +36 14 87-41 00 |
Danmark Bayer A/S Tlf: +45 45 23 50 00 | Malta Alfred Gera and Sons Ltd. Tel: +35 621 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49 (0)214-30 513 48 | Nederland Bayer B.V. Tel: +31-23 – 799 1000 |
Eesti Bayer OÜ Tel: +372 655 8565 | Norge Bayer AS Tlf: +47 23 13 05 00 |
Ελλάδα Bayer Ελλάς ΑΒΕΕ Τηλ: +30-210-61 87 500 | Österreich Bayer Austria Ges.m.b.H. Tel: +43-(0)1-711 46-0 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48 22 572 35 00 |
France Bayer HealthCare Tél (Nº vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351 21 416 42 00 |
Hrvatska Bayer d.o.o. Tel: +385-(0)1-6599 900 | România SC Bayer SRL Tel: +40 21 529 59 00 |
Ireland Bayer Limited Tel: +353 1 216 3300 | Slovenija Bayer d. o. o. Tel: +386 (0)1 58 14 400 |
Ísland Icepharma hf. Sími: +354 540 8000 | Slovenská republika Bayer spol. s r.o. Tel. +421 2 59 21 31 11 |
Italia Bayer S.p.A. Tel: +39 02 397 8 1 | Suomi/Finland Bayer Oy Puh/Tel: +358- 20 785 21 |
Κύπρος NOVAGEM Limited Τηλ: +357 22 48 38 58 | Sverige Bayer AB Tel: +46 (0) 8 580 223 00 |
Latvija SIA Bayer Tel: +371 67 84 55 63 |
Date of last revision of this prospectus:
Other sources of information
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.
If you want local information, scan here to access the website https://www.pi.bayer.com/eylea3.
A QR code with the link to the prospectus is included.
--------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The vial is for single use in one eye only. Extracting multiple doses from a single vial may increase the risk of contamination and subsequent infection.
Do notuse if the packaging or its components have expired, are damaged, or have been tampered with. Check the vial label to ensure you have the intended dose of Eylea. The 8 mg dose requires the use of the Eylea 114.3 mg/ml vial.
Intravitreal injection should be performed with a 30 G × ½ inch (1.27 cm) injection needle (not included).
Using a smaller needle (larger gauge) than the recommended 30 G × ½ inch (1.27 cm) injection needle may cause an increase in injection force.
1. | Before administration, visually inspect the injectable solution. Do notuse the vial if particles, turbidity, or color change are observed. | |
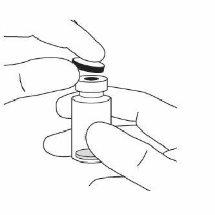
2. | Remove the plastic cap and disinfect the outer part of the vial's rubber stopper. |
|
3. | Use an aseptic technique for steps 3 to 10. Attach the supplied filter needle to a sterile 1 ml syringe with a Luer Lock adapter. |
|
4. | Push the filter needle through the center of the vial stopper until the needle is fully inserted into the vial and its tip comes into contact with the bottom or the lower inner edge of the vial. | |
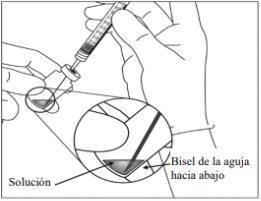
5. | Transfer the entire contents of the Eylea vial to the syringe, keeping the vial in a vertical position and slightly tilted to facilitate complete extraction. To avoid introducing air, ensure the bevel of the filter needle is submerged in the solution. Continue tilting the vial during extraction, keeping the bevel of the filter needle submerged in the solution.
| |
6. | Ensure the plunger rod is sufficiently withdrawn when emptying the vial to completely empty the filter needle. After injection, any unused product must be discarded. | |
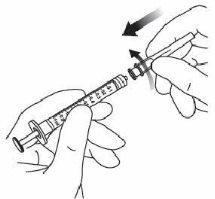
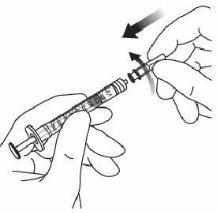
7. | Remove the filter needle and dispose of it properly. Note:the filter needle must notbe used for intravitreal injection. | |
8. | Firmly attach the 30 G × ½ inch (1.27 cm) injection needle to the syringe tip with the Luer Lock adapter by making a rotational movement. |
|
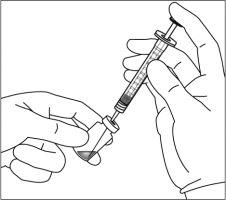
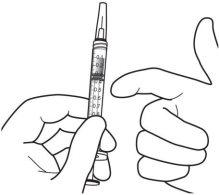
9. | Hold the syringe with the needle pointing upwards and check that there are no bubbles inside. If there are, gently tap the syringe with your finger until they rise to the top. |
|
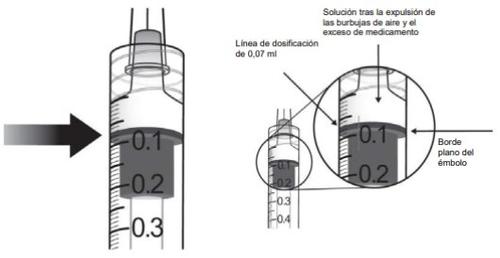
10. | To eliminate all bubbles and to expel excess medication, slowly push the plunger so that the flat edge of the plunger aligns with the line indicating 0.07 mlon the syringe.
|
Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EYLEA 114.3 mg/mL Injectable SolutionDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Celltrion Healthcare Hungary Kft.Prescription required
Online doctors for EYLEA 114.3 mg/mL Injectable Solution
Discuss questions about EYLEA 114.3 mg/mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions