
Eporatio 1,000 IU/0.5 ml Injectable Solution in Pre-filled Syringe

How to use Eporatio 1,000 IU/0.5 ml Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Eporatio 1,000 IU/0.5 ml solution for injection in pre-filled syringe
Eporatio 2,000 IU/0.5 ml solution for injection in pre-filled syringe
Eporatio 3,000 IU/0.5 ml solution for injection in pre-filled syringe
Eporatio 4,000 IU/0.5 ml solution for injection in pre-filled syringe
Eporatio 5,000 IU/0.5 ml solution for injection in pre-filled syringe
Eporatio 10,000 IU/1 ml solution for injection in pre-filled syringe
Eporatio 20,000 IU/1 ml solution for injection in pre-filled syringe
Eporatio 30,000 IU/1 ml solution for injection in pre-filled syringe
Epoetin theta (epoetin theta)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Eporatio and what is it used for
- What you need to know before you use Eporatio
- How to use Eporatio
- Possible side effects
- Storage of Eporatio
- Contents of the pack and other information
- Information for self-injection
1. What is Eporatio and what is it used for
What is Eporatio
Eporatio contains the active substance epoetin theta (epoetin theta), which is almost identical to erythropoietin, a natural hormone produced by the body. Epoetin theta (epoetin theta) is a protein produced by biotechnology. It works in exactly the same way as erythropoietin. Erythropoietin is produced in the kidneys and stimulates the bone marrow to produce red blood cells. Red blood cells are very important for distributing oxygen throughout the body.
What is Eporatio used for
Eporatio is used for the symptomatic treatment of anemia and its symptoms (e.g., fatigue, weakness, and shortness of breath). Anemia occurs when the blood cells do not contain enough red blood cells. Treatment for anemia is given to adult patients with chronic kidney disease or adult patients with non-myeloid cancer (cancer that does not originate in the bone marrow), who are also receiving chemotherapy (cancer medication) at the same time.
2. What you need to know before you use Eporatio
Do not use Eporatio
- if you are allergic to epoetin theta (epoetin theta), other epoetins, or any of the other ingredients of this medicine (listed in section 6);
- if you have uncontrolled high blood pressure.
Warnings and precautions
General
This medicine may not be suitable for the following patients. Please consult your doctor if you belong to any of these patient groups:
- patients with liver problems
- patients with pathological changes in their red blood cells (homocytic sickle cell anemia).
Before and during treatment with this medicine, your blood pressure should be closely monitored. If your blood pressure increases, your doctor may give you medication to lower your blood pressure. If you are already being treated with medication to lower your blood pressure, your doctor may increase the dose. It may also be necessary to reduce the dose of Eporatio or interrupt treatment with Eporatio for a short period.
Consult a doctor immediately if you experience sudden, severe headache, confusion, altered speech, impaired gait, seizures, or convulsions. These may be signs of very high blood pressure, even if your blood pressure is normally normal or low. It is necessary to treat them at the same time.
Your doctor will regularly perform blood tests to monitor various blood components and their levels. Additionally, iron levels in the blood must be monitored before and during treatment with this medicine. If your iron levels are too low, your doctor may also prescribe an iron medication.
If you feel tired and weak or experience fatigue, you should consult your doctor. These symptoms could indicate that treatment with this medicine is ineffective. Your doctor will check that there are no other causes of anemia and may perform blood tests or a bone marrow examination.
The healthcare professional treating you will record exactly which product you are using at all times. This can help provide more information about the safety of medicines like this.
Healthy individuals should not use Eporatio. The use of this medicine by healthy individuals can excessively increase certain blood parameters and cause heart or blood vessel problems, potentially endangering their life.
Severe skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been observed with the administration of epoetins. SJS/TEN can initially appear as red, circular patches, often with central blisters on the trunk. Ulcers in the mouth, throat, nose, genitals, and eyes (eye irritation and swelling) may also appear. These severe skin reactions are often preceded by fever or flu-like symptoms. The skin rash can progress to widespread skin peeling and potentially life-threatening complications. If you present with a severe skin rash or any of these other skin symptoms, stop using Eporatio and contact your doctor or seek immediate medical attention.
Anemia caused by chronic kidney disease
If you are a patient with chronic kidney disease, your doctor will monitor that a certain blood parameter (hemoglobin) does not exceed the defined threshold. If the blood parameters become too high, heart or vascular problems can occur, increasing the risk of death.
If you are a patient with chronic kidney disease and, in particular, if you do not respond adequately to Eporatio, your doctor will monitor your Eporatio dose, as repeated dose increases of Eporatio if you are not responding to treatment can increase the risk of heart or blood vessel problems and may increase the risk of heart attack, stroke, and death.
If you have kidney blood vessel hardening (nephrosclerosis) but do not need to be under dialysis treatment, your doctor will consider whether treatment is suitable for you. In this case, it cannot be excluded with absolute certainty that the progression of kidney function deterioration may be accelerated.
If you are on dialysis, medications that prevent blood clotting are used. If you are being treated with Eporatio, the dose of the anticoagulant medication may need to be increased. Otherwise, the increase in red blood cells can cause blockage of the arteriovenous fistula (an artificial connection between an artery and a vein that is surgically created in dialysis patients).
Anemia in cancer patients
If you are a cancer patient, you should know that this medicine can act as a growth factor for blood cells and, in some circumstances, may have a negative impact on the cancer. Depending on the individual situation, a blood transfusion may be preferable. Please discuss this with your doctor.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age, as there are no data to demonstrate the safety and efficacy of this medicine in this age group.
Other medicines and Eporatio
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
The use of Eporatio in pregnant women has not been investigated. It is important that you inform your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant, as your doctor will decide whether it is advisable not to use this medicine.
It is not known whether the active substance of this medicine passes into breast milk. Your doctor will decide whether it is advisable not to use this medicine while breastfeeding.
Driving and using machines
This medicine does not affect your ability to drive or use machines.
Eporatio contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe; i.e., it is essentially "sodium-free."
3. How to use Eporatio
Your treatment with this medicine will be started by a doctor with experience in the aforementioned indications.
Follow exactly the administration instructions for this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
The recommended dose is…
The dose of Eporatio (expressed in International Units or IU) will depend on your disease, body weight, and the route of injection administration (under the skin [subcutaneous injection] or into a vein [intravenous injection]). Your doctor will indicate the correct dose for you.
Anemia caused by chronic kidney disease
Injections can be administered under the skin or into a vein. Patients on hemodialysis typically receive the injection at the end of dialysis via the arteriovenous fistula. Patients not on dialysis treatment typically receive the injection under the skin. Your doctor will regularly perform blood tests and adjust the dose or suspend treatment if necessary. Hemoglobin values in the blood should not exceed a value of 12 g/dl (7.45 mmol/l). Your doctor will use the lowest effective dose to control the symptoms of anemia. If you do not respond adequately to Eporatio, your doctor will monitor your dose and inform you if it is necessary to change the doses of Eporatio.
Treatment with Eporatio is divided into two parts:
- Correction of anemia
The initial dose for subcutaneous injections is 20 IU per kg of body weight, administered 3 times a week. If necessary, your doctor will increase the dose at monthly intervals.
The initial dose for intravenous injections is 40 IU per kg of body weight, administered 3 times a week. If necessary, your doctor will increase the dose at monthly intervals.
- Maintenance of red blood cell levels
Once an adequate number of red blood cells has been achieved, the maintenance dose required to maintain a constant number will be determined by your doctor.
In the case of subcutaneous injections, the weekly dose may be administered either as 1 injection per week or as 3 divided injections per week.
In the case of intravenous injections, the dose may be adjusted to 2 injections per week.
If you change the frequency of dose administration, a dose adjustment may be necessary.
Treatment with Eporatio is normally long-term treatment.
The maximum dose should not exceed 700 IU per kg of body weight per week.
Anemia in cancer patients
Injections are administered subcutaneously. The injection will be administered once a week. The initial dose is 20,000 IU. Your doctor will regularly perform blood tests and adjust the dose or suspend treatment if necessary. Hemoglobin values in the blood should not exceed a value of 12 g/dl (7.45 mmol/l). You will normally receive Eporatio until 1 month after completing chemotherapy.
The maximum dose should not exceed 60,000 IU.
How are the injections administered?
This medicine is administered by injection using a pre-filled syringe. The injection is administered either into a vein (intravenous injection) or into the tissue just under the skin (subcutaneous injection).
If you receive Eporatio treatment as a subcutaneous injection, your doctor may suggest that you learn to inject this medicine yourself. Your doctor or nurse will give you instructions on how to do this. Do not attempt to administer this medicine yourself without these instructions. Part of the information you need to use the pre-filled syringe can be found at the end of this leaflet (see section 7, "Information for self-injection"). However, for optimal treatment of your disease, close and constant cooperation with your doctor is required.
Each pre-filled syringe is for single use.
If you use more Eporatio than you should
Do not increase the dose that your doctor has prescribed. If you think you have injected more Eporatio than you should, consult your doctor. It is unlikely to be serious. Even with very high levels in the blood, no symptoms of poisoning have been observed.
If you forget to use Eporatio
If you have forgotten an injection or have injected too little, tell your doctor. Do not use a double dose to make up for forgotten doses.
If you stop treatment with Eporatio
Before stopping treatment with this medicine, consult your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects:
- Very high blood pressure:
Consult a doctor immediately if you experience sudden, severe headache, confusion, altered speech, impaired gait, seizures, or convulsions. These may be signs of very high blood pressure (frequent in patients with chronic kidney disease, may affect up to 1 in 10 people), even if your blood pressure is normally normal or low. It is necessary to treat them at the same time.
- Allergic reactions:
Allergic reactions such as skin rash, itching over large areas of skin, severe allergic reactions with weakness, low blood pressure, difficulty breathing, and swelling of the face (frequency not known, cannot be estimated from the available data) have been reported. If you believe you have had this type of reaction, you must stop treatment with Eporatio and seek immediate medical attention.
- Severe skin reactions:
Severe skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been observed with the administration of epoetins. These reactions can appear as red, circular patches, often with central blisters on the trunk, skin peeling, and ulcers in the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms. If you present with these symptoms, stop using Eporatio and contact your doctor or seek immediate medical attention. See also section 2.
You may experience the following additional side effects:
Frequent (may affect up to 1 in 10 people)
- Headache;
- High blood pressure;
- Flu-like symptoms, such as fever, chills, feeling weak, fatigue;
- Skin reactions, such as rash, itching, or reactions at the injection site.
Frequent in patients with chronic kidney disease (may affect up to 1 in 10 people)
- Blood clot in the arteriovenous fistula in patients on dialysis.
Frequent in patients with cancer (may affect up to 1 in 10 people)
- Joint pain.
Frequency not known in patients with chronic kidney disease (cannot be estimated from the available data)
- Cases of a disease called pure red cell aplasia (PRCA) have been reported. PRCA means that the body stops or reduces the production of red blood cells, causing severe anemia. If your doctor suspects or confirms that you have this disease, you should not be treated with Eporatio or any other epoetin.
Frequency not known in patients with cancer (cannot be estimated from the available data)
- Thromboembolic episodes, such as blood clots.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Eporatio
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton and on the pre-filled syringe after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the pre-filled syringe in the outer carton to protect it from light.
You may take Eporatio out of the refrigerator and store it at a temperature not above 25°C for a period of up to 7 days without exceeding the expiry date. Once it is taken out of the refrigerator, the medicine must be used within this time period or discarded.
Do not use this medicine if you notice turbidity or particles in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Eporatio
- The active ingredient is epoetin zeta (epoetin theta).
Eporatio 1,000 UI/0.5 ml: A pre-filled syringe contains 1,000 international units (UI) (8.3 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 2,000 international units (UI) (16.7 micrograms) per ml.
Eporatio 2,000 UI/0.5 ml: A pre-filled syringe contains 2,000 international units (UI) (16.7 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 4,000 international units (UI) (33.3 micrograms) per ml.
Eporatio 3,000 UI/0.5 ml: A pre-filled syringe contains 3,000 international units (UI) (25 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 6,000 international units (UI) (50 micrograms) per ml.
Eporatio 4,000 UI/0.5 ml: A pre-filled syringe contains 4,000 international units (UI) (33.3 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 8,000 international units (UI) (66.7 micrograms) per ml.
Eporatio 5,000 UI/0.5 ml: A pre-filled syringe contains 5,000 international units (UI) (41.7 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 10,000 international units (UI) (83.3 micrograms) per ml.
Eporatio 10,000 UI/1 ml: A pre-filled syringe contains 10,000 international units (UI) (83.3 micrograms) of epoetin zeta (epoetin theta) in 1 ml of injectable solution, equivalent to 10,000 international units (UI) (83.3 micrograms) per ml.
Eporatio 20,000 UI/1 ml: A pre-filled syringe contains 20,000 international units (UI) (166.7 micrograms) of epoetin zeta (epoetin theta) in 1 ml of injectable solution, equivalent to 20,000 international units (UI) (166.7 micrograms) per ml.
Eporatio 30,000 UI/1 ml: A pre-filled syringe contains 30,000 international units (UI) (250 micrograms) of epoetin zeta (epoetin theta) in 1 ml of injectable solution, equivalent to 30,000 international units (UI) (250 micrograms) per ml.
- The other components are sodium dihydrogen phosphate dihydrate, sodium chloride, polysorbate 20, trometamol, hydrochloric acid (6M) (for pH adjustment), and water for injection.
Product Appearance and Container Contents
Eporatio is a clear and colorless injectable solution in a pre-filled syringe with a needle for injection.
Eporatio 1,000 UI/0.5 ml, Eporatio 2,000 UI/0.5 ml, Eporatio 3,000 UI/0.5 ml, Eporatio 4,000 UI/0.5 ml, and Eporatio 5,000 UI/0.5 ml: Each pre-filled syringe contains 0.5 ml of solution.
Packages of 6 pre-filled syringes; 6 pre-filled syringes with safety needles or 6 pre-filled syringes with safety devices.
Eporatio 10,000 UI/1 ml, Eporatio 20,000 UI/1 ml, and Eporatio 30,000 UI/1 ml: Each pre-filled syringe contains 1 ml of solution. Packages of 1, 4, and 6 pre-filled syringes; 1, 4, and 6 pre-filled syringes with safety needles or 1, 4, and 6 pre-filled syringes with safety devices.
Only some package sizes may be marketed.
Marketing Authorization Holder
ratiopharm GmbH
Graf-Arco-Strasse 3
89079 Ulm
Germany
Manufacturer
Teva Biotech GmbH
Dornierstrasse 10
89079 Ulm
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Teva Pharma Belgium N.V./S.A./AG Tel: +32 3 820 73 73 | Lithuania UAB "Sicor Biotech" Tel: +370 5 266 0203 |
Bulgaria Phoenix Pharma Ltd. Tel: +359 2 489 95 85 | Luxembourg ratiopharm GmbH, Germany Tel: +49 731 402 02 |
Czech Republic Teva Pharmaceuticals CR, s.r.o. Tel: +420 251 007 111 | Hungary Teva Gyógyszergyár Zrt. Tel: +36 1 288 64 00 |
Denmark Teva Denmark A/S Tel: +45 44 98 55 11 | Malta Teva Pharmaceuticals Ireland, Ireland Tel: +353 51 321740 |
Germany ratiopharm GmbH Tel: +49 731 402 02 | Netherlands Teva Nederland B.V. Tel: +31 800 0228 400 |
Estonia UAB "Sicor Biotech" Estonian branch Tel: +372 661 0801 | Norway Teva Norway AS Tel: +47 66 77 55 90 |
Greece Teva Ελλάς Α.Ε. Tel: +30 210 72 79 099 | Austria ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1 97 007 |
Spain Teva Pharma, S.L.U. Tel: +34 91 387 32 80 | Poland Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 22 345 93 00 |
France Teva Santé Tel: +33 1 55 91 78 00 | Portugal ratiopharm, Comércio e Indústria de Produtos Farmacêuticos, Lda Tel: +351 21 424 80 00 |
Croatia Pliva Hrvatska d.o.o. Tel: +385 1 37 20 000 | Romania Teva Pharmaceuticals S.R.L Tel: +40 21 230 65 24 |
Ireland Teva Pharmaceuticals Ireland Tel: +353 51 321740 | Slovenia Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Iceland ratiopharm Oy, Finland Tel: +358 20 180 5900 | Slovakia TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 2 57 26 79 11 |
Italy Teva Italia S.r.l. Tel: +39 02 89 17 98 1 | Finland ratiopharm Oy Tel: +358 20 180 5900 |
Cyprus Teva Ελλάς Α.Ε., Greece Tel: +30 210 72 79 099 | Sweden Teva Sweden AB Tel: +46 42 12 11 00 |
Latvia UAB "Sicor Biotech" Latvian branch Tel: +371 673 23 666 | United Kingdom Teva UK Limited Tel: +44 1977 628500 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Information for Self-Injection
This section contains information on how to administer Eporatio injections under the skin. It is essential that you do not attempt to administer an injection without first receiving the necessary instructions from your doctor or nurse. If you are unsure about injecting yourself or have any doubts, consult your doctor or nurse.
How to Administer Eporatio
It should be injected into the tissue just under the skin. This is known as a subcutaneous injection.
Equipment Needed for Administration
To administer the injection under the skin, you will need:
- a pre-filled syringe of Eporatio,
- an alcohol swab,
- a sterile gauze pad or swab,
- a puncture-proof container (plastic container provided by a hospital or pharmacy) to safely dispose of used syringes.
What to Do Before Administering the Injection
- Remove a blister pack containing a pre-filled syringe from the refrigerator.
- Open the blister pack and remove the pre-filled syringe and needle from the pack. Do not touch the pre-filled syringe by the plunger or the syringe cap.
- Check the expiration date on the pre-filled syringe label (CAD). Do not use it if the date is later than the last day of the month shown.
- Check the appearance of Eporatio. It should be a clear and colorless liquid. If there are particles inside or it is cloudy, do not use it.
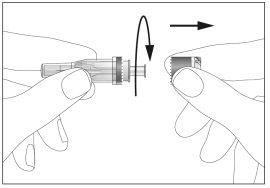
- There is a cap on the end of the needle. Break the security seal and remove the cap (see image 1)
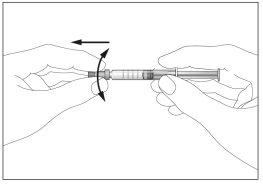
- Remove the cap from the pre-filled syringe (see image 2)
- Attach the needle to the syringe (see image 3). Do not remove the syringe cap yet.
- For a more comfortable injection, let the syringe sit at room temperature (not above 25°C) for 30 minutes or gently hold the pre-filled syringe in your hands for a few minutes. Do not heat Eporatio in any other way (e.g., do not heat it in a microwave or hot water)
- Do not remove the syringe cap until you are ready for injection
- Find a comfortable and well-lit place. Place everything you need within reach (the pre-filled syringe of Eporatio, the alcohol swab, a sterile gauze pad or swab, and a puncture-proof container).
- Wash your hands carefully.
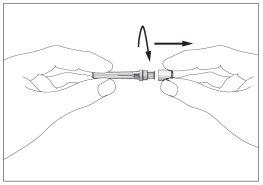
 2
2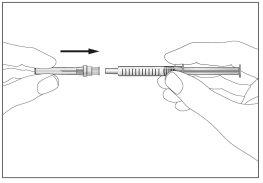 3
3
How to Prepare for the Eporatio Injection
Before administering the Eporatio injection, do the following:
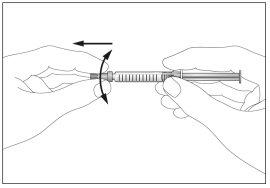
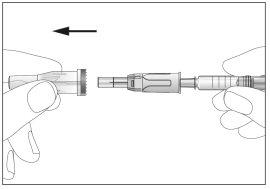
- Take the syringe and carefully remove the protective cover from the needle without tilting it. Separate as indicated in the images 4. Do not touch the needle or push the plunger.
- Small air bubbles may appear in the pre-filled syringe. If there are bubbles, gently tap the syringe with your fingers until the bubbles rise to the top of the syringe. With the syringe pointing upwards, remove all the air from the syringe by pushing the plunger slightly upwards.
- The syringe has a scale on the syringe body. Push the plunger until the number (UI) on the syringe corresponds to the dose of Eporatio prescribed by your doctor.
- Check again that the dose of Eporatio in the syringe is correct.
- You can now use the pre-filled syringe.
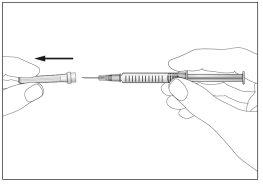 4
4
Where to Inject
The most suitable places for injection are:
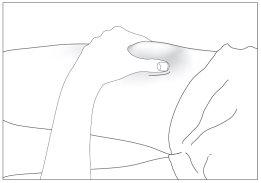
- the top of the thighs,
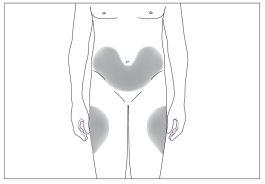
- the abdomen, except for the area around the navel (see the gray areas in image 5).
If someone helps you with the injection, you can also inject into the back and side of the arms (see the gray areas in image 6)
It is best to change the injection site every day to avoid the risk of pain in any of the areas.
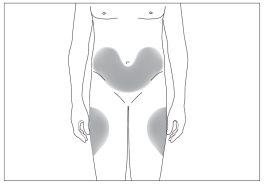 5
5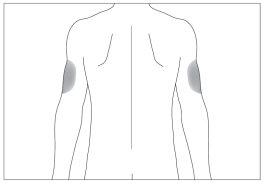 6
6
How to Inject
- Disinfect the injection site using an alcohol swab and pinch the skin between your thumb and index finger, without squeezing (see image 7).
- Insert the needle completely into the skin, as indicated by your doctor or nurse. The angle between the syringe and the skin should not be too small (at least 45°, see image 8).
- Inject the liquid into the tissue slowly and regularly, always keeping the skin pinched.
- After injecting the liquid, remove the needle and pull it out of the skin.
- Press the injection site with a sterile gauze pad or swab for several seconds.
- Use only one syringe for a single injection. Do not use any remaining Eporatio from the syringe.
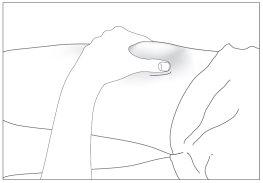 7
7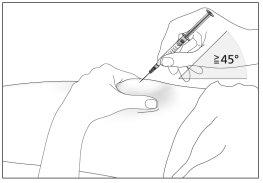 8
8
Remember
If you have any problems, please ask for help or advice from your doctor or nurse.
How to Dispose of Used Syringes
- Do not put the protective cap back on used syringes.
- Place used syringes in a puncture-proof container and keep this container out of sight and reach of children.
- Dispose of the puncture-proof container according to the instructions of your doctor, pharmacist, or nurse.
- Never put used syringes in your household trash
- Information for Self-Injection
This section contains information on how to administer Eporatio injections under the skin. It is essential that you do not attempt to administer an injection without first receiving the necessary instructions from your doctor or nurse. If you are unsure about injecting yourself or have any doubts, consult your doctor or nurse.
How to Administer Eporatio
It should be injected into the tissue just under the skin. This is known as a subcutaneous injection.
Equipment Needed for Administration
To administer the injection under the skin, you will need:
- a pre-filled syringe of Eporatio,
- an alcohol swab,
- a sterile gauze pad or swab.
What to Do Before Administering the Injection
- Remove a blister pack containing a pre-filled syringe from the refrigerator.
- Open the blister pack and remove the pre-filled syringe and needle from the pack. Do not touch the pre-filled syringe by the plunger or the syringe cap.
- Check the expiration date on the pre-filled syringe label (CAD). Do not use it if the date is later than the last day of the month shown.
- Check the appearance of Eporatio. It should be a clear and colorless liquid. If there are particles inside or it is cloudy, do not use it.
- There is a cap on the end of the needle. Break the security seal and remove the cap (see image 1)
- Remove the cap from the pre-filled syringe (see image 2)
- Attach the needle to the syringe (see image 3). Do not remove the syringe cap yet.
- For a more comfortable injection, let the syringe sit at room temperature (not above 25°C) for 30 minutes or gently hold the pre-filled syringe in your hands for a few minutes. Do not heat Eporatio in any other way (e.g., do not heat it in a microwave or hot water)
- Do not remove the syringe cap until you are ready for injection
- Find a comfortable and well-lit place. Place everything you need within reach (the pre-filled syringe of Eporatio, the alcohol swab, and a sterile gauze pad or swab).
- Wash your hands carefully.
 1
1
 2
2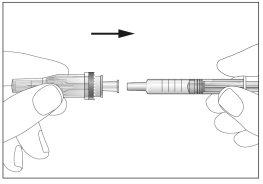 3
3
How to Prepare for the Eporatio Injection
Before administering the Eporatio injection, do the following:
- Take the syringe and carefully remove the protective cover from the needle without tilting it. Separate as indicated in the images 4. The needle is surrounded by a retractable needle protector. Do not touch the needle or the needle protector or push the plunger (see image 5).
- Small air bubbles may appear in the pre-filled syringe. If there are bubbles, gently tap the syringe with your fingers until the bubbles rise to the top of the syringe. With the syringe pointing upwards, remove all the air from the syringe by pushing the plunger slightly upwards.
- The syringe has a scale on the syringe body. Push the plunger until the number (UI) on the syringe corresponds to the dose of Eporatio prescribed by your doctor.
- Check again that the dose of Eporatio in the syringe is correct.
- You can now use the pre-filled syringe.
 4
4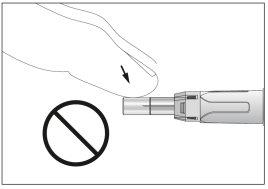 5
5
Where to Inject
The most suitable places for injection are:
- the top of the thighs,
- the abdomen, except for the area around the navel (see the gray areas in image 6).
If someone helps you with the injection, you can also inject into the back and side of the arms (see the gray areas in image 7)
It is best to change the injection site every day to avoid the risk of pain in any of the areas.
 6
6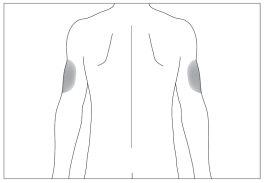 7
7
How to Inject
- Disinfect the injection site using an alcohol swab and pinch the skin between your thumb and index finger, without squeezing (see image 8).
- Insert the protected needle into the skin completely, without hesitation, and with a continuous motion, as indicated by your doctor or nurse. The angle between the syringe and the skin should not be too small (at least 45°, see image 9). The needle protector will retract.
 8
8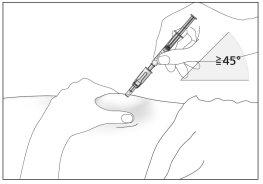 9
9
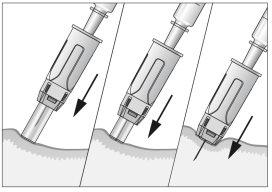
- Aspirate completely when inserting the needle into the skin (see image 10).
- Inject the liquid into the tissue slowly and regularly, always keeping the skin pinched (see image 11).
- After injecting the liquid, remove the needle and pull it out of the skin. The needle will be automatically protected and blocked so that you can prick yourself (see image 12),
- Press the injection site with a bandage pad or a sterile swab for several seconds.
- Use only one syringe for a single injection. Do not use any remaining Eporatio from the syringe.
 8
8 9
9
 10
10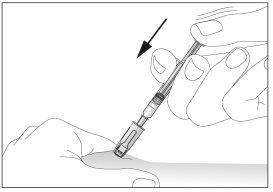 11
11
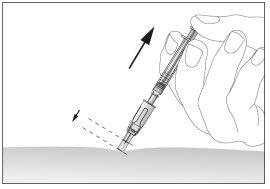 12
12
Remember
If you have any problems, please ask for help or advice from your doctor or nurse.
How to dispose of used syringes
The safety device prevents puncture injuries after use, so no special precautions are required for disposal. Dispose of syringes with safety devices according to the instructions of your doctor, pharmacist, or nurse.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Eporatio 1,000 IU/0.5 ml Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 20000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 20,000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40,000 IU/ml of epoetin alfaActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription required
Online doctors for Eporatio 1,000 IU/0.5 ml Injectable Solution in Pre-filled Syringe
Discuss questions about Eporatio 1,000 IU/0.5 ml Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















