
EMADINE 0.5 mg/ml EYE DROPS SOLUTION


How to use EMADINE 0.5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
EMADINE 0.5mg/mleye drops solution.
emedastine
Read the entire package leaflet carefully before starting to use this medication, as it containsimportant information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is EMADINE and what is it used for
- What you need to know before taking EMADINE
- How to use EMADINE
- Possible side effects
- Storage of EMADINE
- Package contents and additional information
1. What is EMADINE and what is it used for
EMADINE is a medicationfor the treatment of seasonal allergic conjunctivitis in the eyes (allergic eye conditions). It works by reducing the intensity of the allergic reaction.
Allergic conjunctivitis.Certain elements (allergens) such as pollen, house dust, or animal hair can cause allergic reactions that lead to itching and redness, as well as inflammation of the surface of your eyes.
You should consult a doctor if it worsens or does not improve.
2. What you need to know before taking EMADINE
Do not use EMADINE
- If you are allergicto emedastine or any of the other components of this medication (listed in section 6).
Consult your doctor.
Warnings and precautions
- Do not use EMADINE in children under 3years of age.
- If you wear contact lenses, see section "EMADINE contains benzalkonium chloride".
- It is not recommended to use EMADINEin patients over 65 years of age, as this age group has not been studied in clinical trials.
- It is not recommended to use EMADINEin patients with liver or kidney problems.
Consult your doctor or pharmacist before starting to use EMADINE.
Other medications and EMADINE
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
If you use other eye drops at the same time as EMADINE, follow the recommendation at the end of section 3 "How to use EMADINE".
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Driving and using machines
Immediately after applying EMADINE, you may notice that your vision becomes blurry. Do not drive or use machines until your vision is clear.
EMADINE contains benzalkonium chloride
This medication contains 0.5 mg of benzalkonium chloride in every 5 ml, equivalent to 0.1 mg/ml.
The preservative in EMADINE, benzalkonium chloride, can be absorbed by soft contact lenses and may alter their color. Remove your contact lenses before using this medication and wait 15 minutes before putting them back in. Benzalkonium chloride can cause eye irritation, especially if you have dry eyes or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medication.
3. How to use EMADINE
Follow the instructions for administering EMADINE exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is for adults and children over 3 years of age: One drop in the eye, twice a day.
Follow the instructions for administering the medication contained in this package leaflet or as indicated by your doctor. If you are unsure, ask your doctor or pharmacist.
These drops should only be usedin the eyes.
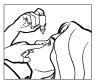
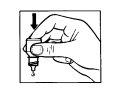
1 2
- Take the EMADINE bottle and stand in front of a mirror.
- Wash your hands.
- Take the bottle and unscrew the cap.
- After opening the bottle for the first time, you must remove the security seal ring before using this medication.
- Hold the bottle, upside down, between your thumb and index finger.
- Tilt your head back. Gently separate the eyelid of the eye with a finger, until a pouch forms, where the drop should fall (Figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye, eyelid, or surrounding areas with the dropper,as the drops remaining in the bottle may become infected.
- Gently press the baseof the bottle to release one drop of EMADINE at a time.
- Do not squeeze the bottle, as it is designed to release a drop with a gentle pressure on the base (Figure 2).
- If you are applying drops to both eyes, repeat the previous steps for the other eye.
- Screw the cap back on the bottle immediately after using the product.
If you accidentally swallow or inject EMADINE, contact your doctor immediately.It may affect your heart rate.
If a drop falls outside the eye,try again.
If you have applied too much,you can remove it by washing your eyes with sterile saline solution or, if not possible, with warm water. Do not apply more drops until it is time for your next dose.
If you forget to apply EMADINEwhen it is due, apply one drop as soon as you remember, and then continue with your usual treatment regimen. Do not apply adouble doseto make up for forgotten doses.
If you are using another eye drop, wait at least 10 minutes between applying EMADINE and the other drops. Ointments should be applied last.
If you have any further questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Unless the effects are severe, continue with the treatment as usual. If these effects concern you, consult your doctor or pharmacist.
Common side effects (may affect up to 1 in 10 people)
- Eye effects: eye pain, itching of the eyes, redness of the eyes
Uncommon side effects (may affect up to 1 in 100 people)
- Eye effects: corneal disorder, abnormal sensation in the eye, increased tear production, tired eyes, eye irritation, blurred vision, corneal spots, dry eye.
- Other effects: headache, difficulty sleeping, headache (around the nasal sinuses), bad taste, rash
Frequency not known (cannot be estimated from available data)
- Other effects: increased heart rate
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of EMADINE
Keep this medication out of the sight and reach of children.
Do not use EMADINE after the expiration date stated on the bottle and carton after "EXP". The expiration date is the last day of the month indicated.
Do not store above 25°C.
To avoid infections, you must discard the bottle fourweeks after opening it for the first time.Note the opening date on the space provided on the carton.
Opening date:
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of EMADINE
- The active ingredient is emedastine 0.5 mg/ml as difumarate.
- The other ingredients are benzalkonium chloride, trometamol, sodium chloride, hypromellose, and purified water. Small amounts of hydrochloric acid and/or sodium hydroxide are added to maintain normal acidity levels (pH levels).
Appearance of the product and package contents
EMADINE is a liquid (a solution) that comes in a carton containing a 5 ml plastic bottle with a screw cap. Only certain pack sizes may be marketed.
Marketing authorization holder
Immedica Pharma AB
SE-113 63 Stockholm
Sweden
Manufacturer
S.A. Alcon-Couvreur N.V.
Rijksweg 14
B-2870 Puurs
Belgium
Manufacturer
Siegfried El Masnou, S.A.
Camil Fabra 58,
08320 El Masnou
Barcelona
Spain
Manufacturer
Immedica Pharma AB
SE-113 63 Stockholm
Sweden
Date of the last revision of this package leaflet:
Other sources of information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Average pharmacy price12.72 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EMADINE 0.5 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Qualix Pharma S.L.Prescription required
Online doctors for EMADINE 0.5 mg/ml EYE DROPS SOLUTION
Discuss questions about EMADINE 0.5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















