ELONVA 100 micrograms injectable solution

How to use ELONVA 100 micrograms injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Elonva 100micrograms solution for injection
Elonva 150micrograms solution for injection
corifollitropin alfa
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Elonva and what is it used for
- What you need to know before you use Elonva
- How to use Elonva
- Possible side effects
- Storage of Elonva
- Contents of the pack and other information
1. What is Elonva and what is it used for
Elonva contains the active substance corifollitropin alfa and belongs to a group of medicines called gonadotropins. Gonadotropins play an important role in fertility and human reproduction. One of these gonadotropins is Follicle Stimulating Hormone (FSH), which is needed in women for the growth and development of follicles (small round sacs in their ovaries that contain eggs) and in adolescent males (from 14 years onwards) for the treatment of delayed puberty due to hypogonadotropic hypogonadism (HH), in combination with a medicine called human chorionic gonadotropin (hCG).
In women
Elonva is used to help achieve pregnancy in women who are undergoing infertility treatment, such as in vitro fertilization (IVF). IVF includes the removal of eggs from the ovary, fertilization of the eggs in the laboratory, and transfer of the embryos to the uterus a few days later. Elonva causes the growth and development of several follicles at the same time through controlled stimulation of the ovaries.
In adolescent males (from 14 years onwards)
Elonva is used to induce the development and function of the testicles and to induce the development of male sexual characteristics in adolescent males with delayed puberty due to HH.
2. What you need to know before you use Elonva
Do not use Elonva:
- if you are allergic (hypersensitive) to corifollitropin alfa or any of the other ingredients of this medicine (listed in section 6)
- if you have cancer of the ovary, breast, uterus, or brain (pituitary or hypothalamus)
- if you have recently had unexpected vaginal bleeding that has not been diagnosed
- if your ovaries do not work due to a disease called primary ovarian insufficiency
- if you have ovarian cysts or enlargement of the ovaries
- if you have malformations of the sex organs that make normal pregnancy impossible
- if you have uterine fibroids that make normal pregnancy impossible
- if you have risk factors for OHSS (OHSS is a serious clinical problem that can occur when the ovaries are overstimulated. It is explained in more detail later in this leaflet.):
- if you have polycystic ovary syndrome (PCOS)
- if you have had OHSS
- if you have previously received a cycle of controlled ovarian stimulation treatment with a result of more than 30 follicles with a size of 11 mm or larger
- if your basal antral follicle count (number of small follicles present in your ovaries at the start of the menstrual cycle) is higher than 20
Warnings and precautions
Talk to your doctor before you start using Elonva.
Ovarian Hyperstimulation Syndrome (OHSS)
Treatment with gonadotropins like Elonva may cause OHSS. This is a condition in which the ovaries are overstimulated and the growing follicles become larger than normal. In rare cases, severe OHSS can be life-threatening. Therefore, it is very important to be closely monitored by your doctor. To check the effects of treatment, your doctor will perform ultrasound scans of your ovaries. Your doctor may also check your hormone levels in blood. (See also section 4).
OHSS can cause fluid to accumulate suddenly in your stomach and chest, which can lead to the formation of blood clots. Tell your doctor immediately if you have:
- severe abdominal swelling and pain in the stomach area (abdomen)
- nausea
- vomiting
- sudden weight gain due to fluid accumulation
- diarrhea
- decreased urine output
- difficulty breathing
You should only use Elonva once during the same treatment cycle, otherwise, the possibility of OHSS may increase.
Before starting treatment with this medicine, tell your doctor if you have ever had OHSS.
Ovarian Torsion
Ovarian torsion is the twisting of an ovary. The twisting of the ovary could cut off the blood flow to the ovary.
Before you start using this medicine, tell your doctor if:
- you have ever had OHSS.
- you are pregnant or think you may be pregnant.
- you have had abdominal surgery.
- you have had a twisted ovary.
- you have cysts or have had cysts on your ovary or ovaries.
Blood Clots (Thrombosis)
Treatment with gonadotropins like Elonva may (like pregnancy) increase the risk of blood clots (thrombosis). Thrombosis is the formation of a blood clot in a blood vessel.
Blood clots can cause serious diseases, such as:
- blockage in your lungs (pulmonary embolism)
- stroke
- heart attack
- blood vessel problems (thrombophlebitis)
- lack of blood flow (deep vein thrombosis), which can cause the loss of your arm or leg.
Talk to your doctor about this before starting treatment, especially if:
- you already know you have an increased risk of thrombosis
- you or a close family member have had thrombosis
- you are significantly overweight.
Multiple Births or Birth Defects
There is an increased chance of having twins or even more than two babies, even when a single embryo is transferred to the uterus. Multiple pregnancies pose an increased risk to the health of both the mother and her babies. Multiple pregnancies and specific characteristics of couples with fertility problems (e.g., the woman's age, certain semen problems, genetic history of both parents) may be associated with an increased likelihood of birth defects.
Pregnancy Complications
If treatment with Elonva results in pregnancy, there is a higher chance of pregnancy outside the uterus (an ectopic pregnancy). Therefore, your doctor should perform an ultrasound scan at the beginning to rule out the possibility of pregnancy outside the uterus.
Ovarian Tumors and Other Reproductive System Tumors
Cases of ovarian tumors and other reproductive system tumors have been reported in women who have undergone infertility treatment. It is not known whether treatment with fertility medicines increases the risk of these tumors in infertile women.
Other Diseases
Also, before you start using this medicine, tell your doctor if:
- you have kidney disease.
- you have uncontrolled pituitary or hypothalamic problems.
- you have underactive thyroid (hypothyroidism).
- your adrenal glands do not work properly (adrenal insufficiency).
- you have high levels of prolactin in your blood (hyperprolactinemia).
- you have any other disease (e.g., diabetes, heart disease, or any other long-term disease).
- your doctor has told you that pregnancy would be dangerous for you.
Using Elonva with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If a pregnancy test is done during your infertility treatment with Elonva, the test may incorrectly indicate that you are pregnant. Your doctor will advise you when you can start doing pregnancy tests. In case of a positive pregnancy test, tell your doctor.
Pregnancy and breastfeeding
Do not use Elonva if you are already pregnant, think you may be pregnant, or are breastfeeding.
Talk to your doctor or pharmacist before using this medicine.
Driving and using machines
Elonva may cause dizziness. If you feel dizzy, do not drive or use machines.
Elonva contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per injectable; this is essentially "sodium-free".
3. How to use Elonva
Follow the instructions for administration of Elonva exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist again.
In women
Elonva is used in women who are undergoing infertility treatment, such as in vitro fertilization (IVF). During this treatment, Elonva is used in combination with a medicine (called a GnRH antagonist) to prevent your ovary from releasing an egg too early. The treatment with the GnRH antagonist usually starts 5-6 days after the injection of Elonva.
It is not recommended to use Elonva in combination with a GnRH agonist (another medicine to prevent your ovary from releasing an egg too early).
In adolescent males (from 14 years onwards)
Elonva, in combination with a medicine called hCG, is used for the treatment of delayed puberty due to HH. Elonva should be administered once every two weeks, in the morning, on the same day of the week.
Dose
Women
In the treatment of women of childbearing age, the dose of Elonva depends on weight and age.
- A single dose of 100 micrograms is recommended in women whose weight is 60 kilograms or less and whose age is up to 36 years inclusive.
- A single dose of 150 micrograms is recommended in women:
- whose weight is more than 60 kilograms, regardless of age.
- from 50 kilograms of weight and older than 36 years of age.
No studies have been conducted in women over 36 years of age with a weight of less than 50 kilograms.
Body weight | ||||
Less than 50 kg | 50 – 60 kg | More than 60 kg | ||
Age | Up to 36 years inclusive | 100 micrograms | 100 micrograms | 150 micrograms |
Over 36 years | Not studied | 150 micrograms | 150 micrograms |
During the first seven days after the injection of Elonva, you should not use Follicle Stimulating Hormone (recombinant) (FSH (rec)). Seven days after the injection of Elonva, your doctor may decide to continue the stimulation cycle with another gonadotropin, such as FSH (rec). This can be continued for a few days until there are enough follicles of adequate size. This can be checked by ultrasound. Then the treatment with FSH (rec) is interrupted and the eggs mature through the administration of hCG (human Chorionic Gonadotropin). The eggs are retrieved from the ovary 34-36 hours later.
In adolescent males (from 14 years onwards)
The dose of Elonva depends on body weight:
For adolescent males with a weight of 60 kg or less
- 100 micrograms of Elonva once every two weeks for 12 weeks, followed by the administration of Elonva (once every 2 weeks) with hCG. If during treatment your body weight increases to more than 60 kg, your doctor may increase your dose of Elonva to 150 micrograms.
For adolescent males with a weight of more than 60 kg
- 150 micrograms of Elonva once every two weeks for 12 weeks, followed by the administration of Elonva (once every 2 weeks) with hCG.
Combination therapy with hCG twice a week (500 - 5000 IU) may be necessary for 52 weeks or more to achieve adult gonadal development.
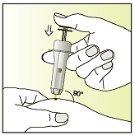
How Elonva is administered
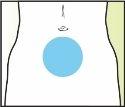
Treatment with Elonva should be supervised by a doctor with experience in the treatment of fertility problems. Elonva should be injected under the skin (subcutaneously) in a skin fold (pinching with your thumb and index finger), preferably just below the navel. The injection can be given by a healthcare professional (e.g., a nurse), your partner, or you yourself, as long as your doctor has carefully instructed you. Always use Elonva exactly as your doctor has told you. You should consult your doctor or pharmacist if you are not sure. At the end of this leaflet, there are step-by-step "instructions for use".
Do not inject Elonva into a muscle.
Elonva comes in pre-filled syringes that have an automatic safety system to help prevent needlestick injuries after use.
If you use more Elonva or FSH (rec) than you should
If you think you have used more Elonva or FSH (rec) than you should, tell your doctor immediately.
If you forget to use Elonva
If you forget to inject Elonva on the day you should, tell your doctor immediately. Do not inject Elonva without consulting your doctor.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
A possible complication of treatment with gonadotropins like Elonva is the unwanted overstimulation of the ovaries. The chance of getting this complication can be reduced by closely monitoring the number of mature follicles. Your doctor will perform ultrasound scans of your ovaries to closely monitor the number of mature follicles. Your doctor may also check your hormone levels in blood. The first symptoms of ovarian overstimulation can be noticed as stomach pain (abdomen), nausea, or diarrhea. Ovarian overstimulation can trigger a condition called Ovarian Hyperstimulation Syndrome (OHSS), which can be a serious clinical problem. In more severe cases, it can cause enlargement of the ovaries, accumulation of fluid in the abdomen and/or chest (which can cause sudden weight gain due to fluid accumulation) or blood clots in the blood vessels.
Tell your doctor immediately if you have stomach pain (abdomen) or any other symptoms of OHSS, even if they occur a few days after the injection.
The chance of getting a side effect is classified into the following categories:
Common (may affect up to 1 in 10 women)
- Ovarian Hyperstimulation Syndrome (OHSS)
- Pelvic pain
- Nausea
- Headache
- Pelvic discomfort
- Breast tenderness
- Fatigue
Uncommon (may affect up to 1 in 100 women)
- Ovarian torsion
- Increased liver enzymes
- Miscarriage
- Pain after egg retrieval
- Pain associated with therapeutic procedure
- Premature ovulation
- Abdominal distension
- Vomiting
- Diarrhea
- Constipation
- Back pain
- Breast pain
- Bruising or pain at the injection site
- Irritability
- Mood swings
- Dizziness
- Hot flashes
Frequency not known (cannot be estimated from the available data)
- Allergic reactions (hypersensitivity reactions, both local and generalized, including skin rash).
Ectopic pregnancies and multiple pregnancies have also been reported. These side effects are not considered to be related to the use of Elonva, but to Assisted Reproductive Technology (ART) or a subsequent pregnancy.
In rare cases, blood clots (thrombosis) have been associated with Elonva therapy, as well as with other gonadotropins, which form in a blood vessel and can break loose and travel in the bloodstream to block another blood vessel (thromboembolism).
If you are an adolescent male
Side effects reported in adolescent males:
Common (may affect up to 1 in 10 males)
- Vomiting
- Pain at the injection site
- Hot flashes
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Elonva
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after “CAD” or “EXP” (expiry date). The expiry date is the last day of the month shown.
Storage by the pharmacist
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Storage by the patient
There are two options:
- Store in a refrigerator (between 2°C and 8°C). Do not freeze.
- Store at a temperature not above 25°C for a period not exceeding one month. Note the date on which you start storing the medicine outside the refrigerator, and use it within one month from that date.
Keep the syringe in the outer packaging to protect it from light.
Do not use Elonva
- If it has been stored outside the refrigerator for more than one month.
- If it has been stored outside the refrigerator at a temperature above 25°C.
- If you notice that the solution is not transparent.
- If you realize that the syringe or needle is damaged.
Empty or unused syringes should not be disposed of in the drains or in the household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package contents and additional information
Composition of Elonva
- The active substance is corifollitropin alfa. Each pre-filled syringe of Elonva 100 micrograms solution for injection contains 100 micrograms in 0.5 milliliters (ml) of solution for injection. Each pre-filled syringe of Elonva 150 micrograms solution for injection contains 150 micrograms in 0.5 milliliters (ml) of solution for injection.
- The other ingredients are: sodium citrate, sucrose, polysorbate 20, methionine, and water for injections. The pH may have been adjusted with sodium hydroxide and/or hydrochloric acid.
Appearance and package contents of the product
Elonva is an aqueous, transparent, and colorless solution for injection in a pre-filled syringe with an automatic safety system that prevents needlestick injuries after use. The syringe is provided with a sterile needle for injection. Each syringe contains 0.5 ml of solution.
The pre-filled syringe is presented in individual packaging.
Elonva is available in two strengths: 100 micrograms and 150 micrograms solution for injection.
Marketing authorization holder and manufacturer
N.V. Organon
Kloosterstraat 6
5349 AB Oss
Netherlands
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Organon Belgium Tel: 0080066550123 (+32 2 2418100) | Lithuania Organon Pharma B.V. Lithuania atstovybe Tel.: +370 52041693 |
Bulgaria Organon Bulgaria EOOD Tel.: +359 2 806 3030 | Luxembourg/Luxemburg Organon Belgium Tel: 0080066550123 (+32 2 2418100) |
Czech Republic Organon Czech Republic s.r.o. Tel: +420 233 010 300 | Hungary Organon Hungary Kft. Tel.: +36 1 766 1963 |
Denmark Organon Denmark ApS Tlf: +45 4484 6800 | Malta Organon Pharma B.V., Cyprus branch Tel: +356 2277 8116 |
Germany Organon Healthcare GmbH Tel.: 0800 3384 726 (+49 (0) 89 2040022 10) | Netherlands N.V. Organon Tel: 00800 66550123 (+32 2 2418100) |
Estonia Organon Pharma B.V. Estonian RO Tel: +372 66 61 300 | Norway Organon Norway AS Tlf: +47 24 14 56 60 |
Greece BIANEΞ Α.Ε. Τηλ: +30 210 80091 11 | Austria Organon Healthcare GmbH Tel: +49 (0) 89 2040022 10 |
Spain Organon Salud, S.L. Tel: +34 91 591 12 79 | Poland Organon Polska Sp. z o.o. Tel.: +48 22 105 50 01 |
France Organon France Tél: +33 (0) 1 57 77 32 00 | Portugal Organon Portugal, Sociedade Unipessoal Lda. Tel: +351 218705500 |
Croatia Organon Pharma d.o.o. Tel: +385 1 638 4530 | Romania Organon Biosciences S.R.L. Tel: +40 21 527 29 90 |
Ireland Organon Pharma (Ireland) Limited Tel: +353 15828260 | Slovenia Organon Pharma B.V., Oss, podružnica Ljubljana Tel: +386 1 300 10 80 |
Iceland Vistor hf. Sími: + 354 535 7000 | Slovakia Organon Slovakia s. r. o. Tel: +421 2 44 88 98 88 |
Italy Organon Italia S.r.l. Tel: +39 06 90259059 | Finland Organon Finland Oy Puh/Tel: +358 (0) 29 170 3520 |
Cyprus Organon Pharma B.V., Cyprus branch Τηλ: +357 22866730 | Sweden Organon Sweden AB Tel: +46 8 502 597 00 |
Latvia Arvalsts komersanta “Organon Pharma B.V.” parstavnieciba Tel: +371 66968876 | United Kingdom (Northern Ireland) Organon Pharma (UK) Limited Tel: +44 (0) 208 159 3593 |
Date of last revision of this leaflet:MM/YYYY
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
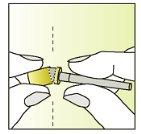
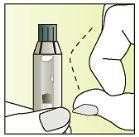
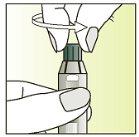
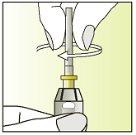
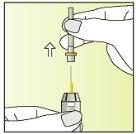
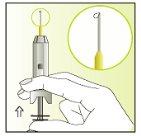
Instructions for use
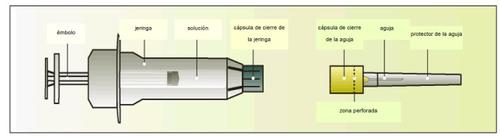
Components of the Elonva syringe and needle
Preparing the injection | |
| 1.
|
| 2.
|
| 3.
|
| 4.
|
| 5.
|
| 6.
|
Injection | |
| 7.
|
| 8.
|
| 9.
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ELONVA 100 micrograms injectable solutionDosage form: INJECTABLE, 150 µgActive substance: corifollitropin alfaManufacturer: Organon N.V.Prescription requiredDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for ELONVA 100 micrograms injectable solution
Discuss questions about ELONVA 100 micrograms injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions