
EKZEM 250 micrograms/ml single-dose ear drops solution


How to use EKZEM 250 micrograms/ml single-dose ear drops solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ekzem 250 micrograms/ml ear drops, solution in single-dose containers
Fluocinolone acetonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.Keep this leaflet. You may need to read it again.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ekzem and what is it used for
- What you need to know before you use Ekzem
- How to use Ekzem
- Possible side effects
- Storage of Ekzem
- Contents of the pack and other information
1. What is Ekzem and what is it used for
Ekzem is a solution for application in the ear. It contains fluocinolone acetonide, a corticosteroid with anti-inflammatory, antipruritic and vasoconstrictor action.
Ekzem is used for the treatment of otic eczema in adults with intact tympanic membrane.
2. What you need to know before you use Ekzem
Do not use Ekzem
- if you are allergic to fluocinolone acetonide, to other corticosteroids or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- This medicine must not be applied in the eye.
- Consult your doctor before using Ekzem if you have or may have any injury (perforation) in the eardrum.
- If, once treatment has started, symptoms of urticaria (itching) or skin rash or any other allergic symptom (e.g. sudden swelling of the face, throat or eyelids, difficulty breathing) occur, stop treatment immediately and consult your doctor. Severe hypersensitivity reactions may require immediate emergency treatment.
- If your doctor also diagnoses a bacterial or fungal infection, you will need to use additional treatment for the infection because if not, it could worsen. In order to reduce adverse reactions, use this medicine at the minimum dose and only for the time advised by your doctor.
Contact your doctor if you experience blurred vision or other visual disturbances.
Use in children and adolescents
The use of fluocinolone acetonide for otic eczema in children and adolescents has not been studied, therefore Ekzem is not recommended for use in this population.
Use of Ekzem with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There are no adequate and well-controlled studies with Ekzem in pregnant women, therefore Ekzem should be used with caution during pregnancy.
Ekzem should also be used with caution in breastfeeding women as it is unknown whether fluocinolone acetonide is secreted in breast milk.
Driving and using machines
Ekzem does not affect the ability to drive or use machines.
3. How to use Ekzem
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is the contents of one ampoule in the affected ear, twice a day, for seven days.
Only use Ekzem in both ears if your doctor has told you to. Your doctor will inform you about the duration of treatment with Ekzem.
Recommendations for use
The person administering Ekzem should wash their hands before starting.
- Separate one ampoule from the strip of ampoules contained in the envelope (image 1).
- Warm the ear drops by holding the ampoule in your hands (image 2).
- Turn the cap on the end of the ampoule once (image 3).
- Tilt your head to one side so that the affected ear is facing upwards (image 4).
- Deposit the entire contents of the ampoule into the affected ear canal (image 5). Gently move the earlobe upwards and outwards. This will allow the ear drops to flow into the ear canal.
- Keep your head tilted for about 1 minute to allow the drops to penetrate the ear (image 6). Discard the ampoule after administration.
- Repeat, if necessary, in the other ear.
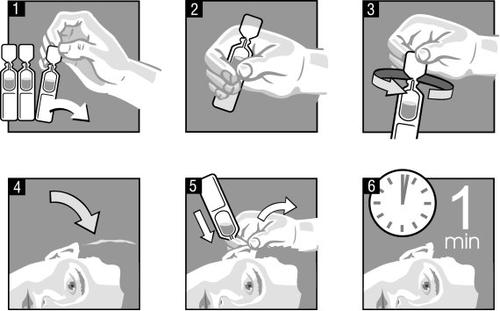
It is important that you follow these instructions to get a good result with this medicine in your ear. Once the medicine has been administered in the ear, it should be kept in the ear for one minute without putting the head in a vertical position or moving it too quickly. This could cause a loss of part of the administered medicine, as the drops may fall down the face and not penetrate the inner part of the ear.
If you use more Ekzem than you should
Symptoms related to overdose are not known. In case of overdose or accidental ingestion, inform your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount administered, or go to the nearest health service.
If you forget to use Ekzem
Do not use a double dose to make up for forgotten doses. Simply continue with the next dose.
If you stop treatment with Ekzem
Do not stop treatment with Ekzem without consulting your doctor or pharmacist. It is very important to use this medicine for the time that your doctor has indicated, even if the symptoms improve.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common (may affect up to 1 in 10 people): burning, itching, irritation, dryness and discomfort in the application area.
Uncommon (may affect up to 1 in 100 people): folliculitis (inflammation of one or more hair follicles), acne, skin discoloration, dermatitis and contact dermatitis (skin inflammation / eczema).
Rare (may affect up to 1 in 1,000 people): skin atrophy (decrease in skin thickness), skin striae, heat erythema and infection.
Frequency not known (cannot be estimated from the available data): blurred vision.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ekzem
Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month stated.
- Store below 25°C and in the original packaging to protect from light.
- Do not use after 3 months of opening the protective aluminum envelope. Keep unused single-dose ampoules in the protective envelope inside the carton.
- Discard the ampoule after administration.
Do not use this medicine if you notice that the product is not a clear, slightly yellowish solution.
Medicines should not be disposed of via wastewater or household waste. Place the empty packaging and any unused medicine in the SIGRE collection point at your usual pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Ekzem
- The active substance is fluocinolone acetonide.
- 1 ml of solution contains 250 micrograms of fluocinolone acetonide.
- Each 0.40 ml ampoule contains 100 micrograms of fluocinolone acetonide.
- The other ingredients are polysorbate 80, glycerol, povidone K90F, lactic acid, sodium hydroxide 1N and purified water.
Appearance and packaging of the product
Ekzem is a clear solution packaged in 0.40 ml single-dose plastic ampoules. The single-dose ampoules are packaged in a protective aluminum envelope and a cardboard box for protection. Each ampoule contains approximately 100 micrograms of fluocinolone acetonide.
Each pack contains 15 or 30 ampoules. Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Laboratorios Salvat, S.A.
C/Gall, 30-36 - 08950
Esplugues de Llobregat
Barcelona - Spain
Manufacturer
INFECTOPHARM GMBH
Von-Humboldt Strabe 1 – 64646
Heppenheim
Germany
or
Laboratorios Salvat, S.A.
C/Gall, 30 -36 – 08950
Esplugues de Llobregat
Barcelona – Spain
or
PHARMALOOP, S.L.
C/Bolivia, 15 – Polígono Industrial Azque
28806 Alcalá de Henares – Madrid (Spain)
This medicine is authorised in the Member States of the European Economic Area under the following names:
Spain Ekzem 250 micrograms/ml ear drops, solution in single-dose containers
Denmark Otazem
Finland Otazem 250 mcg/ml, korvatipat, liuos, kerta-annospakkaus
Germany OtoFlamm
Italy Ekzem
Norway Otazem
Portugal Ekzem, 0.1 mg/ 0.4 ml, gotas auriculares, solução em recipiente unidose
Sweden Otazem 250 mcg/ml, örondroppar, lösning i endosbehållare
Date of last revision of this leaflet:July 2019.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.81 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EKZEM 250 micrograms/ml single-dose ear drops solutionDosage form: OTIC SOLUTION, 3 mg/mlActive substance: ciprofloxacinManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 0.25 mg / 3.49 mgActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 1.2 mg of ciprofloxacin in 0.4 mlActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for EKZEM 250 micrograms/ml single-dose ear drops solution
Discuss questions about EKZEM 250 micrograms/ml single-dose ear drops solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








