
DRACOSAN 1.5 mg/ml ORAL SOLUTION

How to use DRACOSAN 1.5 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Dracosan1.5 mg/ml oral solution
bencidamine hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Follow the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 3 days.
Contents of the Package Leaflet
- What is Dracosan and what is it used for
- What you need to know before taking Dracosan
- How to take Dracosan
- Possible side effects
- Storage of Dracosan
- Contents of the pack and further information
1. What is Dracosan and what is it used for
Bencidamine is an anti-inflammatory agent for the mouth and throat.
Dracosan contains bencidamine hydrochloride, which is used for the local symptomatic relief of pain and irritation of the mouth and throat.
This medicine is used for the local symptomatic relief of pain and irritation of the mouth and throat without fever, in adults and adolescents over 12 years of age.
Dracosan is a ready-to-use solution and is used undiluted for rinsing or gargling for the mouth and throat. In most cases, only a few days are needed to treat acute symptoms of inflammation with this medicine.
If the symptoms worsen or last more than 3 days, you should consult a doctor.
2. What you need to know before taking Dracosan
Do not use Dracosan
- If you are allergic to bencidamine hydrochloride or any of the other components of this medicine (listed in section 6).
- The bencidamine hydrochloride solution should not be used in children under 12 years of age.
Warnings and precautions
Consult your doctor or pharmacist before using this medicine or if you experience any of the following symptoms:
The use of this medicine, especially for prolonged periods, may lead to sensitization phenomena of the treated area. If this occurs, or if you experience any of the side effects mentioned, you should temporarily discontinue the medicine and contact your doctor.
This medicine should be used with caution if you have a history of asthma, as it may cause bronchospasm (sudden feeling of suffocation).
If the pain in the mouth and throat worsens or does not improve within 3 days, you should contact your doctor or dentist. Prolonged use of the medicine may affect the bacterial flora of the mouth.
It is not recommended to use this medicine if you are allergic to salicylic acid or other analgesics called NSAIDs.
Children and adolescents
It should not be used in children under 12 years of age due to the lack of safety and efficacy information.
Other medicines and Dracosan
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
No interactions are known.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. He will decide whether you should use this medicine.
Driving and using machines
This medicine does not affect your ability to drive or use machines.
This medicine contains methyl parahydroxybenzoate
This medicine contains 1 mg/ml of methyl parahydroxybenzoate. Methyl parahydroxybenzoate may cause allergic reactions (possibly delayed).
This medicine contains alcohol (ethanol)
This medicine contains 1.233 mg of alcohol per 15 ml dose, which is equivalent to 82.20 mg/ml of solution (8% w/w).
The amount of this medicine is equivalent to 100 ml of beer or 13 ml of wine.
It is unlikely that the amount of alcohol in this medicine will have any noticeable effect on adults or adolescents. It may have some effects on small children, such as drowsiness.
The amount of alcohol in this medicine may alter the effect of other medicines. Consult your doctor or pharmacist if you are taking other medicines.
If you are pregnant or breastfeeding, consult your doctor or pharmacist before taking this medicine.
If you have an alcohol addiction, consult your doctor or pharmacist before taking this medicine.
This medicine contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per ml; it is essentially "sodium-free".
This medicine contains peppermint oil
Peppermint oil may trigger hypersensitivity reactions (including dyspnea) in sensitized patients.
3. How to use Dracosan
Follow the instructions for administration of this medicine contained in this leaflet or as indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist again.
Adults and adolescents from 12 years:
The recommended dose is 2 to 3 times a day (after meals).
Pour 15 ml of undiluted solution into the measuring cup to rinse the mouth for 20-30 seconds. It is applied by rinsing in case of mouth conditions or by gargling in case of throat conditions.
In case of intense pain, more frequent application is safe (up to 5 times a day).
In most cases, only a few days are needed to treat acute symptoms of inflammation with this medicine.
In most cases, the therapeutic goal is the remission of symptoms of inflammation and the disappearance of pain, especially when swallowing, which is achieved in the first 2-4 days.
There is no risk from prolonged use in cases of stomatitis during irradiation. However, special warnings for prolonged use should be taken into account, as long-term treatment could affect the normal bacterial flora of the oral cavity, and prolonged use of the medicine may lead to sensitization phenomena.
Do not usethe solution continuously for more than 7 days, except if your doctor or dentist indicates otherwise.
Elderly patients:
There are no special dosage recommendations for elderly patients. Unless otherwise prescribed by the dentist or doctor, the dose specified for adults is applied.
Method of administration
For oral use only.
The solution of this medicine is intended for gargling in the mouth and throat and should not be swallowed. Therefore, the solution can only be used for gargling if you can suppress the swallowing reflex and are able to spit out the solution after rinsing or gargling.
Do not eat or drink until one hour after using the medicine.
Instructions for use
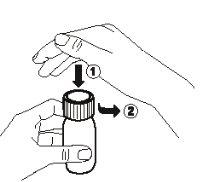
- Open the bottle: press the cap (1) down and turn it counterclockwise (2).
If you use more Dracosan than you should
Involuntary ingestion of small amounts is harmless.
With incorrect use (i.e., if larger amounts of the Dracosan solution are ingested), side effects such as vomiting, abdominal pain, sleep disturbances, restlessness, anxiety, convulsions, ataxia, fever, tachycardia, and possibly respiratory paralysis may occur.
If you have ingested an excessive amount of the medicine or have accidentally swallowed a large amount of it, contact your doctor or pharmacist immediately.
As a first measure, you can try to induce vomiting. Symptomatic treatment is recommended if signs of intoxication appear (e.g., respiratory assistance, elimination of the poison by stomach lavage, etc.).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicological Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Dracosan
If you use too little solution, the time until it starts to take effect may be prolonged, and the treatment may not be effective.
If you forget to use the solution on one occasion, continue with the treatment as recommended without increasing the frequency or dose.
Do not take a double dose to make up for the forgotten dose.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Uncommon(may affect up to 1 in 100 people)
- Redness or sunburn due to increased skin sensitivity to sunlight.
Rare(may affect up to 1 in 1,000 people)
- Burning sensation in the mouth and dry mouth. If this happens, drink a glass of water in sips to reduce the effect.
Very rare(may affect up to 1 in 10,000 people)
- Sudden swelling of the mouth and throat or mucous membranes (angioedema).
- Difficulty breathing (laryngospasm).
Frequency not known(cannot be estimated from the available data)
- Allergic reactions (hypersensitivity)
The symptoms of a severe allergic reaction (anaphylactic shock) may include difficulty breathing (dyspnea), chest pain, chest tightness, dizziness, feeling of weakness, intense itching of the skin, palpable skin nodules, swelling of the face, lips, tongue, and/or throat, and can be potentially fatal.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Dracosan
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the outer packaging and labeling after "EXP". The expiry date is the last day of the month indicated.
Store in the original packaging to protect from light.
After the first opening, use within the next 6 months.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to a pharmacy for disposal. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Dracosan
- The active substance is bencidamine hydrochloride.
1 ml of solution contains 1.5 mg of bencidamine hydrochloride.
- The other components are: methyl parahydroxybenzoate (E 218), ethanol (96%), glycerol (85%), sodium hydrogencarbonate, hydrochloric acid, concentrate (for pH adjustment), polysorbate 20, sodium saccharin (E 954), purified water, peppermint oil (contains: propylene glycol (E 1520), flavoring extracts), quinoline yellow (E 104), patented blue V (E 131).
Appearance of Dracosan and contents of the pack
Green and transparent solution with a typical mint flavor and aroma, presented in a colorless glass bottle with a screw cap. The packaging includes a measuring cup.
Pack sizes: 120 ml, 240 ml, and 300 ml of solution.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Aristo Pharma GmbH
Wallenroder Straße 8-10
13435 Berlin
Germany
You can request more information about this medicine from the local representative of the marketing authorization holder:
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid, Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany Tonsino Gurgellösung bei Halsschmerzen
Italy Tonsino
Spain Dracosan
Poland Nagardlan
Date of the last revision of this leaflet:November 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DRACOSAN 1.5 mg/ml ORAL SOLUTIONDosage form: ORAL SPRAY, 0.51 mg/doseActive substance: benzydamine , benzydamineManufacturer: Angelini Pharma Espana S.L.Prescription not requiredDosage form: TOPICAL ORAL PRODUCT, 0.15 mg benzydamine hydrochloride / 100 mlActive substance: benzydamine , benzydamineManufacturer: Angelini Pharma Espana S.L.Prescription not requiredDosage form: GEL/PASTE/ORAL LIQUID, -Active substance: variousManufacturer: Cooper Consumer Health B.V.Prescription not required
Online doctors for DRACOSAN 1.5 mg/ml ORAL SOLUTION
Discuss questions about DRACOSAN 1.5 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















