
DISNEUMON PERNASAL 5 mg/ml NASAL SPRAY SOLUTION


How to use DISNEUMON PERNASAL 5 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Disneumon pernasal 5 mg/ml nasal spray solution
phenylephrine hydrochloride
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What Disneumon pernasal is and what it is used for
- What you need to know before taking Disneumon pernasal
- How to take Disneumon pernasal
- Possible side effects
- Storage of Disneumon pernasal
- Package contents and additional information
1. What Disneumon pernasal is and what it is used for
The active ingredient of Disneumon pernasal is phenylephrine hydrochloride, which belongs to the group of medications known as nasal decongestants.
Phenylephrine administered nasally has a strong, rapid, and prolonged local vasoconstrictor effect, producing nasal decongestion.
It is indicated for the symptomatic treatment of nasal congestion and secretion due to the common cold or allergic processes, sinusitis, or other upper respiratory tract disorders in adults and children over 12 years of age.
If symptoms persist for more than three days or worsen, consult your doctor.
You should consult a doctor if it worsens or does not improve after 3 days.
2. What you need to know before taking Disneumon pernasal
Do not use Disneumon pernasal
- If you are allergic to phenylephrine or any other component of this medication (listed in section 6).
If you have hypertension or peripheral vascular disease (poor circulation), heart disease, diabetes, hyperthyroidism, Raynaud's syndrome, or prostatic hypertrophy.
If you are being treated with medications for depression (monoamine oxidase inhibitors (MAOIs) or in the two weeks following their withdrawal or tricyclic antidepressants).
If you have recently undergone surgery on the head (if you have undergone any cranial, transnasal, or transoral surgical intervention)
If you have an eye disease with increased pressure (angle-closure glaucoma) or dryness of the nasal mucosa with crust formation (dry rhinitis).
Children under 6 years of age should not use this medication.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Disneumon pernasal.
Consult your doctor before using this medication if:
- you have high blood sugar levels (diabetes)
- you have high blood pressure (hypertension)
- If you suffer from heart disease, including chronic heart diseases, peripheral vascular insufficiency, cardiac rhythm disorders, tachycardia (elevated heart rate), bradycardia (low heart rate), partial cardiac block, angina pectoris;
- If you have a blood vessel disease, such as arteriosclerosis (hardening and thickening of the blood vessel walls);
- If you have poor blood circulation in the brain;
- you have a prostate disease with difficulty urinating (prostatic hypertrophy)
- you have a thyroid disease (hyperthyroidism)
- you have Raynaud's syndrome
- you are taking medications for depression or asthma (from the group called "adrenergic bronchodilators").
- If you have angle-closure glaucoma (a rare eye disease).
In patients with severe heart failure, phenylephrine could worsen heart failure due to blood vessel constriction.
Your blood pressure will be monitored during treatment. If you have heart disease, additional monitoring of your vital functions will be performed.
Do not exceed the recommended dose.
If insomnia appears (very rarely), try to avoid applying the medication in the late afternoon or evening.
If symptoms persist for more than three days or worsen, consult your doctor.
Avoid excessive or continuous use of the medication (usually less than 5 consecutive days), as it may cause rebound congestion with increased nasal congestion and secretion
To avoid contagion, the medication should not be used by more than one person
Athletes are informed that this medication contains a component that may result in a positive doping test.
Children
Consult your doctor to use this medication in children between 6 and 12 years of age.
Do not administer to children under 6 years of age.
Other medications and Disneumon pernasal
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
This medication should not be administered with antidepressants (monoamine oxidase inhibitors - MAOIs - or tricyclic antidepressants, as it may have cardiovascular effects such as hypertension due to the vasoconstrictor effect of phenylephrine) or adrenergic blockers used in the treatment of asthma.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. This medication should not be used during pregnancy.
Breastfeeding women should consult their doctor before using this medication.
Driving and using machines
Not described. If you notice drowsiness or dizziness, do not drive or use machines.
Disneumon pernasal contains sodium
This medication contains less than 23 mg of sodium (1 mmol) per milliliter, which is essentially "sodium-free".
3. How to use Disneumon pernasal
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor, pharmacist, or nurse. If in doubt, ask your doctor, pharmacist, or nurse.
Adults and children over 12 years of age: 1 application in each nostril, which can be repeated every 4-6 hours. Do not shorten the time between applications.
In children between 6 and 12 years of age
one application in each nostril is recommended every 6 hours.
Use in children and adolescents
Children between 6 and 12 years of age
Consult your doctor to use this medication in children between 6 and 12 years of age.
Children under 6 years of age
Do not administer to children under 6 years of age.
Method of administration
Nasal route.
For correct administration of this medication, it is necessary that the bottle and head remain in a vertical position, as indicated in the drawing, while pressing the finger on the top of the diffuser. Each press should be brief, i.e., the necessary time to press and release.
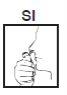
At this moment and simultaneously, a deep inspiration should be made to facilitate the maximum penetration of the medication.
If you tilt the bottle or head while pressing, you may cause the propellant gas to escape, leaving the aerosol without pressure and unusable.
Following these recommendations, it is unlikely that difficulties will arise in the normal use of the medication. However, in some isolated cases, a mechanical failure of the valve or diffuser may occur, preventing the liquid from coming out. If this happens, the product should be replaced with a new one.
If you use more Disneumon pernasal than you should
With high doses or in case of accidental ingestion, unwanted effects such as headaches, nervousness, insomnia, palpitations, hypertension, and blurred vision may appear.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Disneumon pernasal
Do not apply a double dose to make up for forgotten doses.
If you stop treatment with Disneumon pernasal
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Common side effects include headache, sneezing, nasal itching, rhinitis, dryness or burning sensation in the nasal mucosa, and dependence on the medication. The following adverse reactions are uncommon, especially when excessive doses are administered, and may include dizziness, nervousness, tachycardia, dizziness, vomiting, nausea, discomfort, skin rash, hypertension, and palpitations, and difficulty falling asleep.
Excessive or continuous use of the medication may cause rebound congestion with increased nasal congestion and secretion, difficulty urinating, and urinary retention in patients with prostate problems.
If you observe any other adverse reaction not described above, consult your doctor or pharmacist.
Excessive or continuous use of the medication may cause rebound congestion with increased nasal congestion and secretion.
If you observe any other adverse reaction not described above, consult your doctor or pharmacist.
Reporting side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Disneumon pernasal
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the box after CAD or EXP. The expiration date is the last day of the month indicated.
The pressurized container should not be exposed to direct sunlight or temperatures above 50°C.
Do not puncture, break, or burn the container, even if it appears to be empty.
Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Disneumon pernasal
The active ingredient is phenylephrine (in the form of hydrochloride). Each milliliter of solution contains 5 mg of active ingredient.
- The other components (excipients) are: sodium saccharin (E-954), sodium chloride, sodium propionate, menthol aroma, eucalyptol aroma, purified water, and pressurized nitrogen.
Appearance of the product and package contents
Red glass bottle with a white nasal applicator containing 25 ml of nasal spray solution.
Marketing authorization holder
Cooper Consumer Health B.V.
Verrijn Stuartweg 60,
1112 AX Diemen,
Netherlands
Manufacturer
Recipharm Parets, S.L.
Ramón y Cajal, 2, 08150 Parets del Vallés (Barcelona)
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Vemedia Pharma Hispania, S.A.
C/ Aragón, 182, 5th floor
08011 - Barcelona
Spain
Date of the last revision of this package leaflet:April 2020
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DISNEUMON PERNASAL 5 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 1 mgActive substance: xylometazolineManufacturer: Laboratorio De Aplicaciones Farmacodinamicas S.A.Prescription not requiredDosage form: NASAL PRODUCT, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Pharmex Advanced Laboratories S.L.Prescription not requiredDosage form: NASAL PRODUCT, Xylometazoline hydrochloride 0.1%Active substance: xylometazolineManufacturer: Kern Pharma S.L.Prescription not required
Online doctors for DISNEUMON PERNASAL 5 mg/ml NASAL SPRAY SOLUTION
Discuss questions about DISNEUMON PERNASAL 5 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











