
DAPAROX 33 mg/ml ORAL SOLUTION DROPS

How to use DAPAROX 33 mg/ml ORAL SOLUTION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Daparox 33 mg/ml Oral Drops in Solution is and what it is used for
- What you need to know before starting to take Daparox 33 mg/ml oral solution drops
- How to take Daparox 33 mg/ml oral drops in solution
- Possible side effects
- Storage of Daparox 33 mg/ml oral drops in solution
- Package contents and additional information
Introduction
Package Leaflet: Information for the User
Daparox 33 mg/ml Oral Drops in Solution
paroxetine (mesilate)
Read this package leaflet carefully before you start taking this medicine, because it contains important information for you.Keep this package leaflet, you may need to read it again.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What Daparox 33 mg/ml Oral Drops in Solution is and what it is used for
- What you need to know before you start taking Daparox 33 mg/ml Oral Drops in Solution
- How to take Daparox 33 mg/ml Oral Drops in Solution
- Possible side effects
- Storing Daparox 33 mg/ml Oral Drops in Solution
- Package Contents and Additional Information
1. What Daparox 33 mg/ml Oral Drops in Solution is and what it is used for
Paroxetine belongs to a group of antidepressants called selective serotonin reuptake inhibitors (SSRIs).
Daparox is indicated for:
- Major depressive episode (periods of depression)
- Obsessive-compulsive disorder (repetitive obsessive thoughts and/or acts)
- Anxiety disorder with and without agoraphobia (abnormal fear of leaving home, entering
stores, or fear of open spaces)
- Social anxiety disorder/social phobia (exaggerated or avoidance fear of any social
situation)
- Generalized anxiety disorder (generalized fear with much anxiety or nervousness)
2. What you need to know before starting to take Daparox 33 mg/ml oral solution drops
Do not take Daparox if
- you are allergic to paroxetine or any of the other ingredientsof this medicine (listed in section 6).
- you are taking medicines for the treatment of depression or Parkinson's disease (so-called monoamine oxidase inhibitors (MAOIs)).
- You will only be able to use paroxetine if you stop taking irreversible MAOIs at least 14 days in advance (e.g., isocarboxazidand phenelzine).
- If you receive a reversible MAOI (e.g., moclobemide, linezolid, methylthioninium chloride - methylthioninium blue), you should wait at least 24 hours before taking paroxetine.
- Conversely, when you stop taking paroxetine, you should wait at least 7 days before starting to take an MAOI.
- if you are taking a certain medicine (thioridazine) used to treat severe mental illnesses, such as psychosis. Paroxetine may cause an increase in thioridazine levels in the blood, which increases the risk of side effects caused by thioridazine. One of the possible side effects is irregular heartbeats (severe ventricular arrhythmia) and sudden death (see also section 2, "Other medicines and Daparox").
- if you are taking a certain medicine used to treat psychosis (pimozide). Paroxetine may cause an increase in pimozide levels in the blood, which increases the risk of one of the side effects of pimozide (see section 2, "Other medicines and Daparox").
Warnings and precautions
Consult your doctor or pharmacist before starting to take Daparox.
Be especially careful with Daparox
- If you are taking certain medicines used in the treatment of depression or Parkinson's disease (MAOIs). You must not take paroxetine with these medicines.Your doctor will tell you when to start treatment with paroxetine after stopping the administration of these MAOIs (see section 2, "Do not take Daparox", and section 2, "Other medicines and Daparox").
- If you experience symptoms such as restlessness, hyperactivity, or inability to sit still or remain quiet(akathisia). If this is the case, contact your doctor. An increase in dose may be harmful.
- If you start to experience symptoms of serotonin syndrome. This syndrome is characterized by a combination of some of the following symptoms: such as agitation (extreme), confusion, irritability, delirium (hallucinations), sweating, tremors or shivering, increased reflexes and muscle contractions (myoclonus), high fever, stiffness (see section 2, "Other medicines and Daparox"). If you experience any of these symptoms together, contact your doctor immediately and stop taking paroxetine.
- If you have or have had (periods of) extreme euphoria or overexcitement that causes unusual behavior (mania). If you have a manic episode, it may be necessary to discontinue treatment with paroxetine.
- If you have liver or severe kidney problems. Your doctor may need to adjust your dose.
- If you have diabetes. Treatment with paroxetine may alter blood sugar levels, which should be monitored. It may be necessary to adjust the dose of insulin or oral antidiabetics.
- If you have or have had epilepsy or seizures. Paroxetine may cause seizures (apoplexy), so your doctor should pay close attention to this. If you have seizures (apoplexy), contact your doctor immediately. It may be necessary to discontinue treatment with paroxetine.
- If you are receiving electroconvulsive therapy (ECT). So far, the experience with the use of paroxetine during electroconvulsive therapy is limited, so your doctor should pay close attention to this.
- If you have or have had an increase in intraocular pressure(glaucoma). Paroxetine may dilate the pupils (mydriasis), which can lead to an increase in eye pressure, so your doctor should pay close attention to this.
- If you have cardiovascular disorders. The safety of using paroxetine has not been investigated in patients with this disease, so your doctor should exercise extra caution.
- If you are an elderly person, use other medication, or have liver problems (cirrhosis), and as a result, you are at high risk of having low sodium levels in the blood. Paroxetine may decrease sodium levels in the blood, which can cause weakness and fatigue. If this occurs, contact your doctor.
- If you experience an increased tendency to bleed or if you are taking other medicines that may increase the risk of bleeding, or if you are pregnant (see "Pregnancy, breastfeeding, and fertility"). Several examples of these medicines are those used to thin the blood (anticoagulants), certain medicines used to treat severe mental illnesses or nausea and vomiting (phenothiazines), a specific medicine used to treat schizophrenia (clozapine), acetylsalicylic acid, and certain medicines used to alleviate pain and inflammation (NSAIDs, such as ibuprofen or COX-2 inhibitors). Paroxetine may cause abnormal bleeding, so your doctor may need to pay special attention to this (see section 2, "Taking other medicines").
- If you have visual problems. Your doctor will tell you that it is not advisable for you to administer this medicine yourself if you have visual problems. Ask your caregiver or a friend to control the dose you need.
- If you want to stop taking paroxetine, you may experience withdrawal symptoms, particularly if treatment is stopped suddenly (see section 3, If you stop taking Daparox). Consult your doctor before stopping treatment with paroxetine.
Suicidal thoughts and worsening of your depression or anxiety disorder
If you are depressed and/or have an anxiety disorder, you may sometimes have thoughts of harming yourself or suicide. This can increase when you start taking antidepressants, because all these medicines take time to work, usually about two weeks, but sometimes longer.
It is more likely to happen to you:
- If you have had previous thoughts of suicide or self-harm.
- If you are a young adult. Clinical trial data have shown an increased risk of suicidal behavior in adults under 25 with psychiatric disorders who were treated with an antidepressant.
If you have thoughts of self-harm or suicide at any time, contact your doctor or go to the hospital immediately.
It may be useful for you to explain to a family member or close friendthat you are depressed or have an anxiety disorder, and ask them to read this leaflet. You can also ask them to tell you if they think your depression or anxiety is getting worse, or if they are worried about changes in your behavior.
Some medicines in the same group as Daparox (called SSRIs/SNRIs) may cause symptoms of sexual dysfunction (see section 4). In some cases, these symptoms persist after stopping treatment.
Children and adolescents
Paroxetine should not be used in children and adolescents under 18 years of age. You should know that in patients under 18, there is an increased risk of adverse effects such as suicide attempts, suicidal thoughts, and hostility (especially aggression, confrontational behavior, and anger) when taking this class of medicines. Nevertheless, your doctor may prescribe paroxetine to patients under 18 when they decide it is the most convenient for them. If the doctor has prescribed paroxetine to a patient under 18 and you want to discuss this decision, go see them. You should inform your doctor if any of the symptoms mentioned earlier appear or worsen in patients under 18 treated with paroxetine. Additionally, it has not been shown with certainty whether this medicine affects growth, maturity, and cognitive or behavioral development in this age group.
Other medicines and Daparox
Tell your doctor or pharmacistif you are taking, have recently taken, or might take any other medicines.
There are other medicines whose effects may be affected by paroxetine. In turn, these medicines may affect the efficacy of paroxetine. Paroxetine may interact with the following medicines:
- Medicines used for the treatment of depression or Parkinson's disease (MAOIssuch as moclobemideor isocarboxazid), a dietary supplement (L-tryptophan), medicines for migraine (triptans, such as sumatriptan, almotriptan), analgesics (tramadol, buprenorphine, pethidine), a medicine used against infections (linezolid), a reversible non-selective MAOI that acts as a preoperative visualizing agent (methylthioninium chloride - methylthioninium blue), other selective serotonin reuptake inhibitors (SSRIs, such as fluoxetine, sertraline), medicines used for the treatment of psychiatric disorders (lithium, risperidone), a medicine used for the treatment of chronic pain or anesthesia (fentanyl), and St. John's Wort(Hypericum perforatum), a herbal remedy for depression. The simultaneous use of these medicines can produce serotonin syndrome (see section 2, "Do not take Daparox" and section 2, "Warnings and precautions").
- Buprenorphinecombined with naloxone, substitution treatment for opiate addiction.
- Treatment of psychosis (pimozide). Studies on the concomitant use of paroxetine and pimozide show that paroxetine can increase the amount of pimozide in the blood. Since pimozide can cause serious side effects such as heart rhythm disorders, you should not use paroxetine at the same time as pimozide (see section 2, "Do not take Daparox").
- Medicines inhibiting enzymes, such as certain medicines used for the treatment of depression (clomipramine). Your doctor will probably prescribe a lower dose than usual. If you are going to use paroxetine with enzyme inducers (e.g., carbamazepine, rifampicin, phenobarbital, and phenytoin), you will not usually need a lower initial dose, and your doctor will adjust the following doses according to the effect of the medicine.
- Due to the interaction with paroxetine, there may be a prolongation of the muscle relaxant effect of muscle relaxants used in anesthesia, such as mivacurium and suxamethonium.
- Combination of medicines for treating AIDS (Acquired Immune Deficiency Syndrome)(fosamprenavirand ritonavir).
- Medicines used for the treatment of Parkinson's disease (procyclidine). The effects and side effects of procyclidine may increase. If you start to notice side effects such as dry mouth, blurred vision, constipation, and urinary retention in the bladder due to a disorder of emptying, it may be necessary to reduce the dose of procyclidine, after consulting your doctor.
- Certain medicines for the treatment of epilepsy(anticonvulsants such as sodium valproate). Although it has not been shown to have a direct effect, your doctor should prescribe paroxetine with great care in patients with epilepsy.
- Medicines that are broken down by the same liver enzymes as paroxetine, such as certain medicines for depression (tricyclic antidepressants, such as nortriptylineand desipramine), certain medicines for severe mental illnesses, such as antipsychotics (perphenazine, thioridazine, and risperidone), a medicine used to treat attention deficit hyperactivity disorder (ADHD) (atomoxetine), certain medicines for heart rhythm disorders (such as flecainideand propafenone), certain medicines for the treatment of chest tightness (angina pectoris) and high blood pressure (metoprolol), a medicine used to treat high cholesterol levels (pravastatin), and certain medicines for the treatment of severe mental illnesses or nausea and vomiting (phenothiazines). The effect and side effects of these medicines may increase. You should not use paroxetine and thioridazine together, because of the risk of serious side effects such as heart rhythm disorders (severe ventricular arrhythmia) and sudden death (see section 2, "Do not take Daparox").
- Anticoagulant tablets (anticoagulants such as acenocoumarol, phenprocoumon). The effect and side effects of these medicines may increase, as well as the risk of bleeding. Your doctor should monitor you more closely and may need to adjust the dose of anticoagulants (see section 2, "Warnings and precautions").
- Medicines used in lung cancer or fertility problems (tamoxifen).
- Medicines that increase the risk of bleeding. Certain medicines used to treat severe mental illnesses or nausea and vomiting (phenothiazines, such as chlorpromazine, perphenazine), a medicine used to treat schizophrenia (clozapine), certain medicines used to treat depression (tricyclic antidepressants, such as clomipramine, desipramine), acetylsalicylic acid, medicines used for pain and inflammation (NSAIDssuch as ibuprofenor COX-2 inhibitors, such as rofecoxib, celecoxib) (see section 2, "Warnings and precautions").
- Medicines used to reduce stomach acidity (cimetidine, omeprazole).
Using Daparox with food, drinks, and alcohol
Consuming alcohol and paroxetine at the same time should be avoided.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
There is not enough data to determine the safety and efficacy of using paroxetine during pregnancy.
Some studies have shown an increased risk of heart defects in newborns of mothers who received paroxetine in the first months of pregnancy. You and your doctor can decide whether it is better to change to another treatment or gradually stop treatment with paroxetine. However, depending on the circumstances, your doctor may advise you that it is best to continue treatment.
Make sure your midwife and/or doctor know that you are taking paroxetine. If you take Daparox in the final stage of pregnancy, there may be a higher risk of heavy vaginal bleeding shortly after delivery, especially if you have a history of bleeding disorders. Your doctor or midwife should know that you are taking Daparox to advise you.
Medicines like paroxetine may increase the risk of a serious disease in babies, called persistent pulmonary hypertension of the newborn (PPHN), when taken during pregnancy, particularly at the end of pregnancy, making the baby breathe faster and appear blue. If this happens to your baby, you should contact your midwife and/or doctor immediately. If you take paroxetine during the last 3 months of pregnancy, your newborn baby may also have other conditions, which start during the first 24 hours after delivery. These include sleep problems and feeding problems, respiratory problems, blue discoloration, temperature changes, vomiting, persistent crying, muscle stiffness or flexibility, apathy, tremors, nervousness, or irritability. If the baby has any of these symptoms when born, contact your doctor or midwife, who will advise you.
Paroxetine passes into breast milk in small amounts. If you are taking paroxetine, talk to your doctor before starting breastfeeding. You and your doctor can decide whether you can breastfeed while taking paroxetine.
In animal studies, it has been shown that paroxetine reduces sperm quality. In theory, this could affect fertility, although its impact on human fertility is not yet known.
Driving and using machines
Paroxetine does not affect the ability to drive or use machines. However, this medicine can cause side effects (such as blurred vision, dizziness, drowsiness, or confusion). If you notice any of these side effects, do not drive or operate tools or machines, or perform any other activity that requires you to be alert or concentrated. This means that before performing such activities, you should observe your reaction to paroxetine.
Daparox contains ethanol (alcohol), propylene glycol, and sodium
This medicine contains 67 mg of ethanol in each 20 drops, which corresponds to an amount of 111 mg/ml (11% p/v). The amount in 20 drops is equivalent to less than 2 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine will not have any noticeable effect.
This medicine contains 490 mg of propylene glycol in each 20 drops, which is equivalent to 811 mg/ml.
This medicine contains less than 23 mg of sodium (1 mmol) per ml; that is, it is essentially "sodium-free".
3. How to take Daparox 33 mg/ml oral drops in solution
Follow the administration instructions for this medication exactly as indicated by your doctor. Consult your doctor or pharmacist if you have any doubts.
 Paroxetine should be taken preferably in the morning with food.
Paroxetine should be taken preferably in the morning with food.
Take paroxetine with water, never with another type of drink.
Paroxetine can be administered using a dropper (dose of 10 mg to 30 mg) or an oral syringe (dose of 40 mg to 60 mg).
If your doctor recommends using the dropper, pour the necessary number of drops into a glass of water (200 ml), stir the mixture well, and drink the entire contents of the glass.
To avoid errors when counting 40 drops or more, your doctor may consider prescribing this medication in tablet form or using the syringe to administer the oral suspension (the dose is expressed in ml).
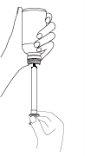
If your doctor recommends using an oral syringe, insert the syringe tip into the plastic dropper of the bottle, turn the bottle upside down, and load the number of ml that has been prescribed into the syringe. Discharge the contents of the syringe into a glass of water (200 ml), mix well, and drink the entire glass.
After each use, rinse the syringe with water and let it air dry.
The recommended dose is as follows:
- Major depressive episode
The recommended dose is 20 mg (20 drops) daily. Under normal conditions, you should start to feel better within a period of one week, although the effects may appear later (such as after two weeks). If the effects are insufficient, your doctor may gradually increase the dose at intervals of 10 mg (10 drops) up to a maximum of 50 mg (1.5 ml) daily. Your doctor will determine the period during which you must continue taking the drops, which may last more than 6 months.
- Obsessive-compulsive disorder (OCD)
The recommended dose is 40 mg (1.2 ml) daily, with an initial dose of 20 mg (20 drops) daily. If the effects are insufficient, your doctor may gradually increase the dose at intervals of 10 mg (10 drops). The maximum daily dose is 60 mg (1.8 ml). Your doctor will determine the period during which you must continue taking the drops, which may last a few months or even longer.
- Anxiety disorder with or without agoraphobia
The recommended dose is 40 mg (1.2 ml) daily, with an initial dose of 10 mg (10 drops) daily. If the effects are insufficient, your doctor may gradually increase the dose at intervals of 10 mg (10 drops). The maximum daily dose is 60 mg (1.8 ml). The initial dose is low to avoid worsening the symptoms of the anxiety disorder at the start of treatment. Your doctor will determine the period during which you must continue taking the drops, which may last a few months or even longer.
- Social anxiety disorder/social phobia
The recommended dose is 20 mg (20 drops) daily. If the effects are insufficient, your doctor may gradually increase the dose at intervals of 10 mg (10 drops). The maximum daily dose is 50 mg (1.5 ml). Your doctor will determine the period during which you must continue taking the drops, which may last a long time, and during this interval, the treatment should be periodically assessed.
- Generalized anxiety disorder
The recommended dose is 20 mg (20 drops) daily. If the effects are insufficient, your doctor may gradually increase the dose at intervals of 10 mg (10 drops). The maximum daily dose is 50 mg (1.5 ml). Your doctor will determine the period during which you must continue taking the drops, which may last a long time, and during this interval, the treatment should be periodically assessed.
Elderly patients
The recommended initial dose for elderly patients is the same as the initial dose in other adult patients, but the maximum daily dose should not exceed 40 mg (1.2 ml).
Use in children and adolescents
Paroxetine should not be used in children or adolescents under 18 years of age (see section 2, "Children and adolescents").
Patients with renal or hepatic impairment
If you have liver or severe kidney problems, your doctor should adjust your dose.
Duration of treatment
Your doctor will determine the period during which you must continue taking paroxetine. Depending on your illness, you may need to take paroxetine for a long time. You should continue taking paroxetine for some time, even when your symptoms have subsided, to avoid their reappearance. Do not stop treatment with paroxetine without consulting your doctor.If you suddenly stop treatment with paroxetine, you may experience withdrawal symptoms, so the dose should be gradually reduced (see section 3, "If you stop treatment with Daparox").
If you take more Daparox than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20 (indicating the medication and the amount ingested). In addition to the known side effects (see section 4, "Possible side effects"), you may experience the following symptoms: fever and involuntary muscle contractions.
If you forget to take Daparox
Do not take a double dose to make up for forgotten doses. Omit the forgotten dose and take the next one when it is due. If you have any doubts, always consult your doctor.
If you stop treatment with Daparox
Do not stop treatment with Daparox without consulting your doctor, and never stop treatment suddenly, as this may cause withdrawal symptoms.
The effects you may experience if you stop taking paroxetine are: dizziness, sensory disturbances [tingling or prickling sensation, sensation of electric shocks, buzzing, whistling, hissing, ringing, or other persistent noises in the ears (tinnitus)], anxiety, sleep disturbances (such as vivid dreams or nightmares), and headache. Less common effects include: excitement, nausea, tremors, confusion, sweating, emotional instability, visual disturbances, strong heartbeats (palpitations), diarrhea, and irritability (see section 4, Possible side effects).
These symptoms usually start in the first few days after stopping treatment but can also occur in patients who forget to take a dose. Normally, the withdrawal effects disappear within two weeks. In some patients, they can be more severe or can last longer (2-3 months or more). If you and your doctor decide to stop treatment with paroxetine, the daily dose should be gradually reduced over a few weeks or months (starting with doses of 10 mg per week). You should always consult your doctor before reducing the dose.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Contact your doctor or go to the hospital immediately if you experience any of the following side effects during treatment.
Side effects uncommon(may affect up to 1 in 100 people)
- abnormal bleeding, predominantly skin hematomas and gynecological bleeding.
Side effects rare(may affect up to 1 in 1,000 people)
- seizures and convulsions
- feeling restless and hyperactive with inability to sit or stay still (akathisia)
- low sodium levels in the blood (hyponatremia), predominantly in elderly patients.
Side effects very rare(may affect up to 1 in 10,000 people)
- allergic reactions, which can be severewith paroxetine, including itching and painful skin rash (urticaria) or severe reactions that cause swelling of the skin, throat, or tongue, difficulty breathing and/or itching (angioedema). If you develop a red and lumpy rash, swelling of the eyelids, face, lips, mouth, or tongue, start to feel itchy or have difficulty breathing (shortness of breath) or swallowing, and feel weak or dizzy and, consequently, fall or lose consciousness, contact your doctor or go to the hospital immediately.
- serotonin syndrome (symptoms may include agitation, confusion, sweating, hallucinations, hyperreflexia, sudden muscle contractions (myoclonus), tremors, and increased heart rate (tachycardia).
- sudden increase in eye pressure (acute glaucoma)
Side effects with unknown frequency(cannot be estimated from the available data)
- aggression, cases of self-harm/thoughts or behaviors have been reported during treatment with paroxetine or immediately after stopping treatment. However, these symptoms can also be due to the underlying disease.
- heavy vaginal bleeding shortly after childbirth (postpartum hemorrhage), see "Pregnancy, breastfeeding, and fertility" in section 2 for more information.
Other side effects
Side effects very common(may affect more than 1 in 10 people)
- nausea
- sexual dysfunction such as ejaculation problems, decreased sexual desire, impotence in men, and inability to achieve orgasm.
Side effects common(may affect up to 1 in 10 people)
- increased cholesterol levels in the blood, decreased appetite.
- drowsiness, inability to sleep (insomnia), agitation, abnormal dreams (including nightmares).
- dizziness, tremors, headache, loss of concentration (decreased concentration)
- blurred vision
- yawning
- constipation, diarrhea, vomiting, dry mouth
- sweating
- weight gain, feeling of general weakness with loss of muscle strength (asthenia).
Side effects uncommon(may affect up to 1 in 100 people)
- decreased white blood cell count
- if you are a diabetic patient, you may notice a loss of control of your blood sugar levels while taking paroxetine. Consult your doctor about adjusting the dose of insulin or diabetes medication.
- confusion, imagining things that are not really there (hallucinations)
- uncontrolled body or face movements (extrapyramidal disorders)
- pupil dilation (mydriasis)
- rapid heart rate (sinus tachycardia)
- feeling of weakness or dizziness when standing up (postural hypotension)
- skin rash, itching (pruritus)
- problems emptying the bladder (urinary retention) and urinary incontinence
Side effects rare(may affect up to 1 in 1,000 people)
- euphoria or overexcitement, causing abnormal behavior (mania, manic episodes), anxiety, panic attacks, loss of personality
- irresistible urge to move the legs (Restless Legs Syndrome)
- slow heart rate (bradycardia)
- elevated liver enzymes
- muscle pain (myalgia), joint pain (arthralgia)
- high levels of the hormone prolactin in the blood (hyperprolactinemia), which can cause abnormal milk production in the breast in men and women (galactorrhea) and menstrual disorders (including heavy or irregular periods, bleeding between periods, and absence or delay of menstruation).
Side effects very rare(may affect up to 1 in 10,000 people)
- reduction of blood platelets, with increased risk of bleeding or bruising (thrombocytopenia)
- fluid retention and low sodium levels in the blood as a result of the syndrome of inadequate antidiuretic hormone secretion (SIADH)
- gastrointestinal bleeding
- liver disorders such as inflammation (hepatitis), sometimes associated with jaundice and/or liver failure
- severe skin effects (including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis), urticaria, sensitivity to sunlight.
- pain in the erection (priapism)
- swelling of the arms and/or legs (peripheral edema)
Side effects with unknown frequency(cannot be estimated from the available data)
- teeth grinding
- buzzing, whistling, or other persistent noises in the ears (tinnitus)
- colon inflammation (causing diarrhea)
Patients taking this medication have a higher risk of bone fractures.
Withdrawal symptoms observed when stopping treatment with paroxetine
Common: dizziness, sensory disturbance, sleep disturbance, anxiety, and headache.
Uncommon: excitement, nausea, sweating, tremors, confusion, emotional instability, visual disturbance, palpitations, irritability, and diarrhea.
These symptoms are usually mild to moderate and disappear on their own. Do not stop treatment with paroxetine without consulting your doctor and never stop treatment suddenly, as you may experience withdrawal symptoms (see section 3, "If you stop treatment with Daparox").
Additional side effects in children and adolescents
When children and adolescents under 18 years of age received paroxetine, at least 1 in 100, but less than 1 in 10 children/adolescents experienced one of the following side effects: Emotional changes (crying and mood changes), self-harm, suicidal thoughts and attempts, hostile and unfriendly behavior, loss of appetite, tremors, abnormal sweating, hyperactivity, excitement, nausea, stomach pain, and nervousness.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects that do not appear in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Daparox 33 mg/ml oral drops in solution
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date indicated on the packaging after "EXP". The expiration date is the last day of the month indicated.
This medication does not require any special storage conditions.
After opening, the solution should be used within 56 days.
Medications should not be thrown away through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Daparox 33 mg/ml oral drops in solution
- The active ingredient is paroxetine (in the form of mesylate).
1 ml of Daparox contains 33.1 mg of paroxetine (in the form of mesylate).
One drop contains 1 mg of paroxetine (in the form of mesylate).
- The other ingredients (excipients) are:
Sodium saccharin (E954)
Acesulfame K (E950)
Peppermint flavor (peppermint essential oil, menthol, eucalyptol, ethanol, water)
Polysorbate 80 (E433)
Ethanol (111 mg/ml)
Propylene glycol (E1520).
Appearance of Daparox and package contents
Daparox is a clear, reddish-brown to light brown solution packaged in a 20 ml amber glass bottle containing at least 18.5 ml of solution.
The bottle is packaged in a cardboard box and contains a dropper and a child-resistant closure. It may also include an oral syringe.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Holder:
ANGELINI PHARMA ESPAÑA, S.L.
c/ Antonio Machado, 78-80.
3rd floor, module A-Australia Building
08840 Viladecans, Barcelona (Spain)
Phone: 932 534 500
Manufacturer:
Synthon BV
Microweg 22
6545 CM Nijmegen
Netherlands
Synthon Hispania, S.L.
- Castelló, 1
Polígono Industrial Las Salinas
08830 Sant Boi de Llobregat (Barcelona) - Spain
Hormosan Pharma GmbH
Hanauer Landstrasse 139 - 143
60314 Frankfurt am Main - Germany
This medication is authorized in the member states of the European Economic Area under the following names
Austria: Ennos 33.1 mg/ml, Lösung zum Einnehmen
Germany: Paroxetine-Hormosan 33.1 mg/ml.
Italy: Dapagut 33.1 mg/ml, gocce orali, soluzione
Netherlands: Parmite
Spain: Daparox 33 mg/ml gotas orales en solución
Date of the last revision of this leaflet: December 2023
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price7.18 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DAPAROX 33 mg/ml ORAL SOLUTION DROPSDosage form: TABLET, 20 mgActive substance: paroxetineManufacturer: Angelini Pharma Espana S.L.Prescription requiredDosage form: TABLET, 20 mgActive substance: paroxetineManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: TABLET, 20 mg paroxetine hydrochlorideActive substance: paroxetineManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for DAPAROX 33 mg/ml ORAL SOLUTION DROPS
Discuss questions about DAPAROX 33 mg/ml ORAL SOLUTION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











