CYSTAGON 150 mg HARD CAPSULES

How to use CYSTAGON 150 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
CYSTAGON 50 mg Hard Capsules
CYSTAGON 150 mg Hard Capsules
cysteamine bitartrate (mercaptamine bitartrate)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is CYSTAGON and what is it used for
- What you need to know before you take CYSTAGON
- How to take CYSTAGON
- Possible side effects
- Storage of CYSTAGON
- Contents of the pack and further information
1. What is Cystagon and what is it used for
Cystinosis is a metabolic disease called 'nephropathic cystinosis' characterized by the abnormal accumulation of the amino acid cystine in various organs of the body such as the kidneys, eyes, muscles, pancreas, and brain. The accumulation of cystine causes damage to the kidneys and the elimination of excessive amounts of sugar (glucose), proteins, and electrolytes. At different ages, different organs may be affected.
CYSTAGON is prescribed to treat this rare hereditary disorder. CYSTAGON is a medicine that reacts with cystine, reducing its levels in cells.
2. What you need to know before you take Cystagon
Do not take CYSTAGON:
- if you or your child are allergic to cysteamine bitartrate or penicillamine or any of the other ingredients of this medicine (listed in section 6).
- if you are pregnant, this is especially important during the first trimester
- if you are breastfeeding.
Warnings and precautions:
- When your disease or your child's disease has been confirmed by measurements of white blood cell cystine, treatment with CYSTAGON should be started as soon as possible.
- Some cases of skin lesions in the form of small hard bumps, mainly on the elbows, have been reported in children treated with high doses of different cysteamine preparations. These lesions were associated with stretch marks on the skin and bone lesions, such as fractures and bone deformities, and with loose joints.
Your doctor may periodically ask you for physical examinations of the skin and X-rays of the bones to monitor the effects of the medicine. Self-examination of the skin by the patient or parents is recommended. If any skin or bone abnormality appears, you should inform your doctor immediately.
- Your doctor may periodically ask you for blood tests to monitor your blood cell count.
- CYSTAGON does not prevent the accumulation of cystine crystals in the eyes. If you have used eye drops containing cysteamine for this purpose, you should continue using them...
- Unlike phosphocysteamine, another active substance similar to cysteamine bitartrate, CYSTAGON does not contain phosphate. You may be taking phosphate supplements, and it is possible that your doctor may adjust the dose of these supplements when you switch from CYSTAGON to phosphocysteamine.
- The capsules should not be given to children under the age of approximately 6 years to avoid the risk of aspiration into the lungs.
- Do not ingest the desiccant container in the bottle.
Other medicines and CYSTAGON
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
CYSTAGON with food and drinks:
For children under six years of age, the hard capsule can be opened and its contents sprinkled on food (such as milk, potatoes, or starchy foods like flour), or mixed with the bottle. Do not mix with acidic drinks, such as orange juice. For complete instructions, consult your doctor.
Pregnancy
Do not use CYSTAGON during pregnancy. You should consult your doctor if you plan to become pregnant.
Breastfeeding
CYSTAGON should not be used during breastfeeding.
Driving and using machines:
CYSTAGON may cause drowsiness. When starting treatment, you or your child should avoid engaging in potentially hazardous activities until you are well aware of the effects of the medicine.
3. How to take Cystagon
Follow your doctor's instructions for taking this medicine exactly. If you are in doubt, consult your doctor again.
The dose of CYSTAGON prescribed for you or your child will depend on their weight and age.
For children up to 12 years of age, the dose will be based on body size (surface area), with the usual dose being 1.30 g/m2 of body surface area per day.
For patients over 12 years of age and weighing more than 50 kg, the usual dose is 2 g/day.
In any case, the usual dose should not exceed 1.95 g/m2/day.
CYSTAGON should always be taken orally and exactly as instructed by your doctor or your child's doctor. To ensure that CYSTAGON works properly, it is necessary:
- To follow the doctor's instructions exactly. Do not increase or decrease the amount of medicine without the doctor's approval.
- Not to give the hard capsules to children under the age of 6 because they may not swallow them and may choke. For children under 6 years of age, the hard capsule can be opened and its contents sprinkled on food (such as milk, potatoes, or starchy foods like flour), or mixed with the bottle. Do not mix with acidic drinks, such as orange juice. For complete instructions, consult your doctor.
- The medical treatment prescribed for you or your child will include, in addition to CYSTAGON, one or more supplements to replace the important electrolytes eliminated by the kidneys. It is essential to take or give these supplements exactly as instructed. If you forget several doses of one of the supplements, or if you notice weakness or drowsiness, you should consult your doctor immediately.
- To determine the correct dose of CYSTAGON, it is necessary to have periodic blood tests to measure the amount of cystine inside the white blood cells. Your doctor or your child's doctor will determine when the blood tests should be performed. It is also necessary to perform periodic blood and urine tests to measure the concentrations of important electrolytes in the body, so that your doctor can adjust the doses of the electrolyte supplements you need.
CYSTAGON should be taken 4 times a day, every 6 hours, preferably with meals or immediately after meals. It is essential to respect the rule of taking the dose every six hours as strictly as possible.
Treatment with CYSTAGON should be continued indefinitely as indicated by your doctor.
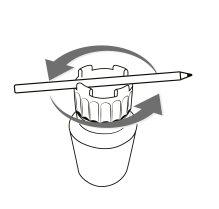
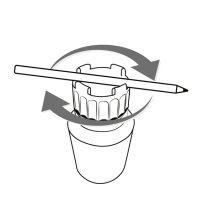
To easily open and close the Cystagon bottle, follow the instructions below:
Instructions for opening and closing the container
OPEN
| CLOSE
|
To open the container Place a pen or similar object between the raised parts of the cap and turn it in the indicated direction (counterclockwise) | To close the container Place a pen or similar object between the raised parts of the cap and turn it in the indicated direction (clockwise) |
If you take more CYSTAGON than you should
If you have taken more medicine than prescribed, or if drowsiness occurs, you should consult your doctor or your child's doctor immediately, or go to the emergency department of a hospital.
If you forget to take CYSTAGON
If you have forgotten to take a dose of the medicine, take it as soon as possible. However, if it is less than 2 hours before the next dose, you should skip the missed dose and return to the normal schedule. Do not take a double dose to make up for the missed doses.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
CYSTAGON may cause drowsiness or decreased alertness in some people. Make sure you know how you or your child reacts to this medicine before engaging in any activity that may be hazardous if you are not alert.
The following side effects have been reported:
- Very common (affects at least 1 in 10 people): vomiting, nausea, diarrhea, loss of appetite, fever, and drowsiness.
- Common (affects at least 1 in 100 people): abdominal pain or discomfort, bad breath and body odor, skin rash, gastroenteritis, fatigue, headache, encephalopathy (brain disorder), and changes in liver function tests.
- Uncommon (affects at least 1 in 1,000 people): stretch marks on the skin, skin lesions (small hard bumps on the elbows), loose joints, leg pain, bone fractures, scoliosis (spinal curvature), bone deformities and fragility, hair depigmentation, severe allergic reaction, drowsiness, seizures, nervousness, hallucinations, decreased white blood cell count, gastrointestinal ulcer with secondary digestive bleeding, and kidney problems that manifest with swelling of the limbs and weight gain.
Since some of these side effects are serious, ask your doctor or pediatrician to explain the warning signs.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cystagon
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C.
Keep the container tightly closed to protect it from light and moisture.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of CYSTAGON
- The active substance is cysteamine bitartrate (mercaptamine bitartrate). Each hard capsule of CYSTAGON 50 mg contains 50 mg of cysteamine (as mercaptamine bitartrate). Each hard capsule of CYSTAGON 150 mg contains 150 mg of cysteamine (as mercaptamine bitartrate).
- The other ingredients are microcrystalline cellulose, pregelatinized starch, magnesium stearate/sodium lauryl sulfate, colloidal silicon dioxide, sodium croscarmellose. The capsule shells: gelatin, titanium dioxide, black ink in the hard capsules (E172).
Appearance and packaging
Hard capsules
- Cystagon 50 mg: White opaque hard capsules with the markings CYSTA 50 on the body and RECORDATI RARE DISEASES on the cap.
Bottles of 100 or 500 hard capsules. Not all pack sizes may be marketed.
- Cystagon 150 mg: White opaque hard capsules with the markings CYSTAGON 150 on the body and RECORDATI RARE DISEASES on the cap.
Bottles of 100 or 500 hard capsules. Not all pack sizes may be marketed.
Marketing authorization holder
Recordati Rare Diseases
Immeuble “Le Wilson”
70, Avenue du Général de Gaulle
F-92800 Puteaux
France
Manufacturer
Recordati Rare Diseases
Immeuble “Le Wilson”
70, Avenue du Général de Gaulle
F-92800 Puteaux
France
or
Recordati Rare Diseases
Eco River Parc
30, rue des Peupliers
F-92000 Nanterre
France
You can obtain further information on this medicine by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien Recordati Tél/Tel: +32 2 46101 36 | Lietuva Recordati AB. Tel: + 46 8 545 80 230 Švedija |
| Luxembourg/Luxemburg Recordati Tél/Tel: +32 2 46101 36 Belgique/Belgien |
Ceská republika Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francie | Magyarország Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Franciaország |
Danmark Recordati AB. Tlf : +46 8 545 80 230 Sverige | Malta Recordati Rare Diseases Tel: +33 1 47 73 64 58 Franza |
Deutschland Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 | Nederland Recordati Tel: +32 2 46101 36 België |
Eesti Recordati AB. Tel: + 46 8 545 80 230 Rootsi | Norge Recordati AB. Tlf : +46 8 545 80 230 Sverige |
Ελλ?δα Recordati Rare Diseases Τηλ: +33 1 47 73 64 58 Γαλλ?α | Österreich Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 Deutschland |
España Recordati Rare Diseases Spain S.L.U. Tel: + 34 91 659 28 90 | Polska Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francja |
France Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 | Portugal Jaba Recordati S.A. Tel: +351 21 432 95 00 |
Hrvatska Recordati Rare Diseases Tél: +33 (0)1 47 73 64 58 Francuska | România Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Franta |
Ireland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Slovenija Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francija |
Ísland Recordati AB. Simi:+46 8 545 80 230 Svíþjóð | Slovenská republika Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 Francúzsko |
Italia Recordati Rare Diseases Italy Srl Tel: +39 02 487 87 173 | Suomi/Finland Recordati AB. Puh/Tel : +46 8 545 80 230 Sverige |
Κ?προς Recordati Rare Diseases Τηλ : +33 1 47 73 64 58 Γαλλ?α | Sverige Recordati AB. Tel : +46 8 545 80 230 |
Latvija Recordati AB. Tel: + 46 8 545 80 230 Zviedrija | United Kingdom (Northern Ireland) Recordati Rare Diseases UK Ltd. Tel: +44 (0)1491 414333 |
Date of last revision of this leaflet:07/2024
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CYSTAGON 150 mg HARD CAPSULESDosage form: CAPSULE, 50 mgActive substance: mercaptamineManufacturer: Recordati Rare DiseasesPrescription requiredDosage form: CAPSULE, 25 mgActive substance: mercaptamineManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: CAPSULE, 75 mgActive substance: mercaptamineManufacturer: Chiesi Farmaceutici S.P.A.Prescription required
Online doctors for CYSTAGON 150 mg HARD CAPSULES
Discuss questions about CYSTAGON 150 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions