
CYSTADROPS 3.8 mg/ml EYE DROPS SOLUTION

How to use CYSTADROPS 3.8 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Cystadrops 3.8 mg/ml Eye Drops Solution
cysteamine (mercaptamine)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Cystadrops and what is it used for
- What you need to know before you use Cystadrops
- How to use Cystadrops
- Possible side effects
- Storage of Cystadrops
- Contents of the pack and other information
1. What is Cystadrops and what is it used for
What is Cystadrops
Cystadrops is an eye drop solution that contains the active substance cysteamine (also known as mercaptamine).
What is it used for
It is used to reduce the amount of cystine crystals on the surface of the eye (cornea) in adults and children over 6 months of age with cystinosis.
What is cystinosis
Cystinosis is a rare inherited disease in which the body is unable to remove excess cystine (an amino acid), leading to the accumulation of cystine crystals in various organs (such as kidneys and eyes). The accumulation of crystals in the eye can lead to increased sensitivity to light (photophobia), deterioration of the cornea (keratopathy), and vision loss.
2. What you need to know before you use Cystadrops
Do not use Cystadrops
If you are allergic to cysteamine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before you start using Cystadrops.
Other medicines and Cystadrops
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
Although the level of Cystadrops in the blood is insignificant, precautions should be taken.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
A few minutes after using Cystadrops, you may notice that your vision becomes blurry. Do not drive or use machines until your vision clears.
Cystadrops contains benzalkonium chloride
This medicine contains 5 micrograms of benzalkonium chloride per drop, equivalent to 0.1 mg/ml.
Benzalkonium chloride can be absorbed by soft contact lenses, altering their color. Remove your contact lenses before using this medicine and wait 15 minutes before putting them back in.
Benzalkonium chloride can cause eye irritation, especially if you have dry eyes or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you notice any unusual sensation in the eye, itching, or eye pain after using this medicine.
3. How to use Cystadrops
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Recommended dose
- The recommended dose is 1 drop in each eye, 4 times a day during waking hours.
- The recommended interval between each application is 4 hours (e.g., you can use the eye drops at 8 am, 12 pm, 4 pm, and 8 pm).
- To avoid sticky eyes in the morning, advise the patient to apply the last drop at least 30 minutes before bedtime.
- Your doctor may gradually reduce the dose (down to a minimum daily dose of 1 drop in each eye) depending on the results of the eye examination.
Use the eye drops only in your eyes (via the ophthalmic route).
To use the eye drops, follow these instructions carefully.These instructions are also available in a video that you can find at www.cystadrops.net. ‘The QR code should be included’
Step 1: Before using a vial for the first time
- The patient should keep Cystadrops at room temperature before the first administration. This will make it easier to use the drops.
- Immediately before using a vial for the first time, write the opening date on the space provided on the carton.
- Wash your hands carefully to avoid bacterial contamination of the vial contents.
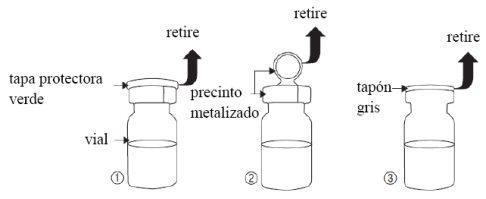
- Remove the green protective cap (figure 1).
- Remove the metal seal (figure 2).
- Remove the gray stopper (figure 3) from the vial.
- Do not touch the vial opening after removing the gray stopper.
- Take the dropper out of its bag without touching the end that will be inserted into the vial, insert it (figure 4) into the vial. Do not remove the dropper from the vial.
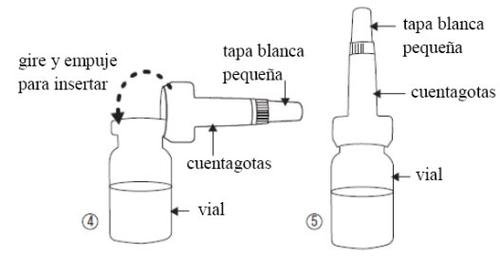
- Do not lose the small white cap (figure 5) that comes on top of the dropper.
Step 2: Before using the eye drops
- Check the opening date you wrote on the carton. Cystadrops can be used for a maximum of 7 days from the moment of opening.
- Take the dropper vial and stand in front of a mirror.
- Wash your hands.
Step 3: Using the eye drops
- Hold the dropper vial upside down, between your thumb and other fingers. Move the dropper vial firmly up and down to facilitate filling the dropper.
- Unscrew the small white cap from the dropper.
- Tilt your head back. Gently pull the lower eyelid down with a clean finger, until a pocket forms between the eyelid and the eye. This is the place to apply the eye drops (figure 6).
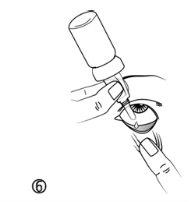
- Bring the tip of the dropper vial close to the eye. You can use a mirror if it helps.
- Do not touch the eye, eyelid, or surrounding areas with the dropper.This could infect the eye drops.
- Gently squeeze the dropper to release one drop of Cystadrops at a time. Be careful not to touch the tip of the dropper with your fingers.
- After using Cystadrops, press the edge of the eye, next to the nose (figure 7), then gently massage the upper eyelid to spread the eye drops over the eye.
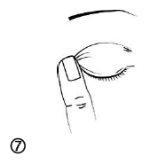
- To avoid possible irritation, remove excess medication around the eye with a damp cloth (figure 8).
- Repeat step 3 with the other eye.
- Put the small white cap back on the dropper immediately after use.
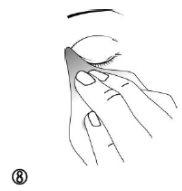
Step 4: Storage of the eye drops after use
- Keep the dropper vial in the carton.
- Keep Cystadrops at room temperature (to facilitate the use of the dropper).
- Discard 7 days after the first opening.Use a new vial.
If a drop does not fall into the eye
Try again.
If you use Cystadrops with another eye medicine
Wait 10 minutes between using Cystadrops and the other eye medicine. Administer eye ointments last.
If you wear soft contact lenses
Do not use the eye drops with the lenses in. After using the eye drops, wait 15 minutes before putting the lenses back in.
If you use more Cystadrops than you should
If you have applied too much eye drops, rinse your eyes preferably with saline solution (if not possible, with lukewarm water). Do not apply more drops until it is time for your next dose.
If you forget to use Cystadrops
Wait until your next application and then continue with your usual treatment schedule. Do not use a double dose to make up for forgotten doses.
If you stop using Cystadrops
Cystadrops should be used daily for the medicine to work properly. If you stop using Cystadrops, it may increase the accumulation of cystine crystals in the eye (cornea) and lead to increased sensitivity to light (photophobia), deterioration of the cornea (keratopathy), and vision loss. Therefore, consult your doctor before stopping this treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Normally, you can continue to use the eye drops unless you have severe side effects. If these side effects worry you, talk to your doctor or pharmacist. Do not stop using Cystadrops without consulting your doctor first.
The following side effects have been reported:
Very common side effects(may affect more than 1 in 10 people)
- eye pain
- redness, itching, or irritation of the eyes (burning)
- tearing
- blurred vision
- discomfort at the site where the eye drops were applied (mainly sticky eyes and eyelashes), accumulation of the medicine on the eyelashes or around the eyes
Common side effects(may affect up to 1 in 10 people)
- abnormal sensation in the eye, feeling of having something in the eye
- dry eyes
- swollen eyelid
- eyelid irritation
- visual disturbance
- pain at the site where the eye drops were applied
- stye
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cystadrops
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP.
Before opening:
- Store in a refrigerator (between 2°C and 8°C).
- Keep the vial in the outer packaging to protect it from light.
After the first opening:
- Write the opening date of the vial on the space provided on the carton.
- Cystadrops can be used for a maximum of 7 days from the moment of opening.
- Keep the dropper vial tightly closed in the outer packaging to protect it from light.
- Store below 25°C.
- Do not refrigerate.
- Discard the dropper vial 7 days after the first opening, even if it is not empty.Use a new vial.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Cystadrops
- The active substance is cysteamine (mercaptamine), in the form of hydrochloride. One milliliter of eye drop solution contains 3.8 mg of cysteamine.
- The other ingredients are benzalkonium chloride (see section 2, in ‘Cystadrops contains benzalkonium chloride’), disodium edetate, sodium carmellose, citric acid monohydrate, sodium hydroxide, hydrochloric acid, and water for injections.
Appearance and packaging of the product
Cystadrops is a clear and viscous eye drop solution.
Each carton contains:
- 1 amber glass vial with 5 ml of eye drop solution,
- 1 dropper.
Cystadrops is available in a pack containing 1 carton or in a multipack containing 4 cartons.
Not all pack sizes may be marketed in your country.
Marketing authorisation holder
Recordati Rare Diseases
Tour Hekla 52 Avenue du Général de Gaulle
92800 Puteaux
France
Manufacturer
Recordati Rare Diseases
Tour Hekla 52 Avenue du Général de Gaulle
92800 Puteaux
France
or
Recordati Rare Diseases
Eco River Parc
30, rue des Peupliers
F-92000 Nanterre
France
You can request more information about this medicine from the local representative of the marketing authorisation holder:
Belgium Recordati Tel: +32 2 46101 36 | Lithuania Recordati AB. Tel: +46 8 545 80 230 Sweden |
| Luxembourg Recordati Tel: +32 2 46101 36 Belgium |
Czech Republic Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Hungary Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Denmark Recordati AB. Tel: +46 8 545 80 230 Sweden | Malta Recordati Rare Diseases Tel: +33 1 47 73 64 58 France |
Germany Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 | Netherlands Recordati Tel: +32 2 46101 36 Belgium |
Estonia Recordati AB. Tel: +46 8 545 80 230 Sweden | Norway Recordati AB. Tel: +46 8 545 80 230 Sweden |
Greece Recordati Rare Diseases Tel: +33 1 47 73 64 58 France | Austria Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 Germany |
Spain Recordati Rare Diseases Spain S.L.U. Tel: +34 91 659 28 90 | Poland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
France Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 | Portugal Recordati Rare Diseases SARL Tel: +351 21 432 95 00 |
Croatia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Romania Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Ireland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Slovenia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Iceland Recordati AB. Tel: +46 8 545 80 230 Sweden | Slovakia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Italy Recordati Rare Diseases Italy Srl Tel: +39 02 487 87 173 | Finland Recordati AB. Tel: +46 8 545 80 230 Sweden |
Cyprus Recordati Rare Diseases Tel: +33 1 47 73 64 58 France | Sweden Recordati AB. Tel: +46 8 545 80 230 |
Latvia Recordati AB. Tel: +46 8 545 80 230 Sweden |
Date of the last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CYSTADROPS 3.8 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Online doctors for CYSTADROPS 3.8 mg/ml EYE DROPS SOLUTION
Discuss questions about CYSTADROPS 3.8 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















