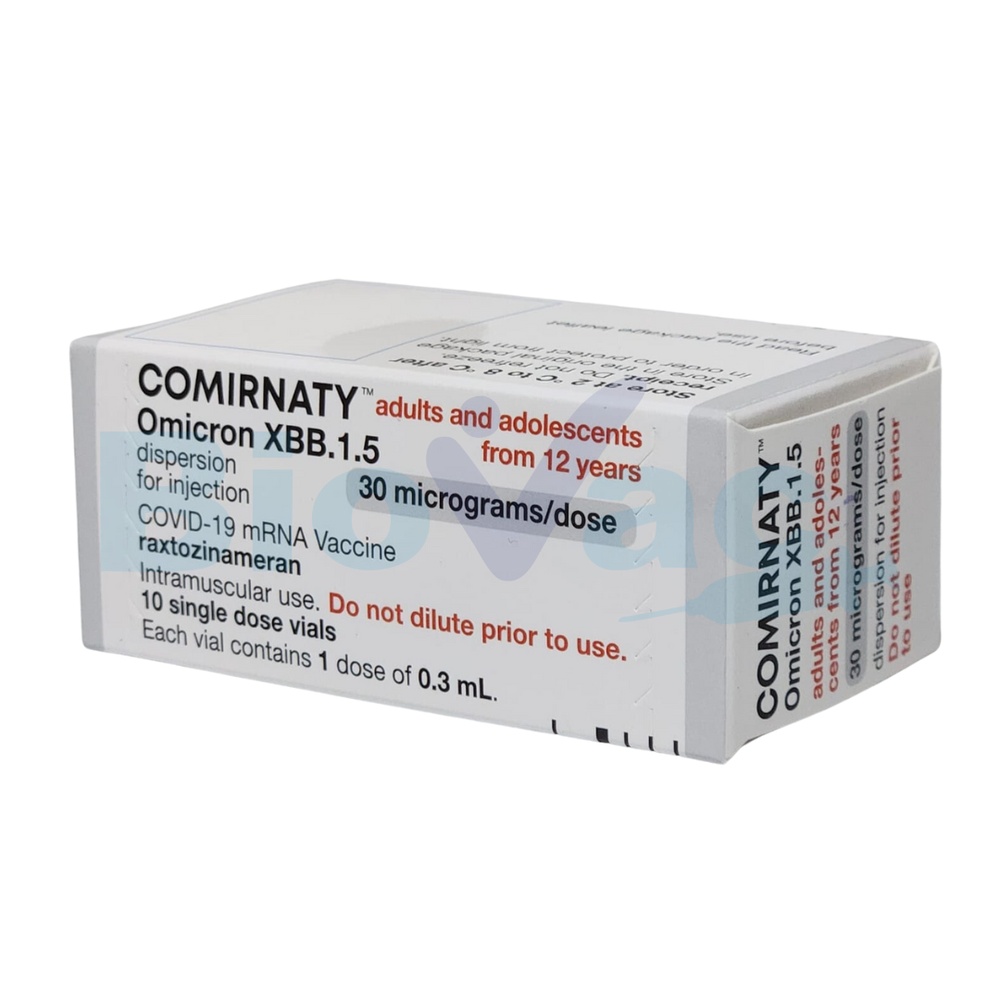
COMIRNATY 30 micrograms/dose Injectable Dispersion

How to use COMIRNATY 30 micrograms/dose Injectable Dispersion
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Comirnaty 30 micrograms/dose dispersion for injection
Adults and adolescents from 12 years of age
COVID-19 mRNA vaccine (with modified nucleosides)
tozinameran
This medicine is subject to additional monitoring, which will allow for quicker identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you receive this vaccine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Comirnaty and what is it used for
- What you need to know before you receive Comirnaty
- How Comirnaty is administered
- Possible side effects
- Storage of Comirnaty
- Contents of the pack and further information
1. What is Comirnaty and what is it used for
Comirnaty is a vaccine used to prevent COVID-19 caused by SARS-CoV-2.
Comirnaty 30 micrograms/dose dispersion for injection is administered to adults and adolescents from 12 years of age.
The vaccine makes the immune system (the body's natural defenses) produce antibodies and blood cells that fight the virus, providing protection against COVID-19.
Because Comirnaty does not contain the virus to produce immunity, it cannot give you COVID-19.
2. What you need to know before you receive Comirnaty
Comirnaty must not be administered
- if you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before receiving the vaccine if:
- you have ever had a severe allergic reaction or breathing problems after receiving any other vaccine or after receiving Comirnaty in the past;
- you are nervous about the vaccination process or have fainted after an injection with a needle;
- you have a severe illness or infection with high fever. However, you can be vaccinated if you have a mild fever or an upper respiratory tract infection such as a cold;
- you have a bleeding disorder, bruise easily, or are taking a medicine to prevent blood clots;
- you have a weakened immune system due to a disease such as HIV or due to a medicine, such as corticosteroids, that affects the immune system.
There is a higher risk of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the outer lining of the heart) after vaccination with Comirnaty (see section 4). These disorders can occur within a few days of vaccination and have mainly occurred within 14 days. They have been observed more frequently after the second dose of vaccination and more frequently in young males. The risk of myocarditis and pericarditis appears to be lower in children between 5 and 11 years of age than in those between 12 and 17 years of age. After vaccination, you should be alert to the signs of myocarditis and pericarditis, such as difficulty breathing, palpitations, and chest pain, and should seek immediate medical attention if they occur.
As with any vaccine, Comirnaty may not fully protect all people who receive it, and it is not known how long you will be protected.
You may receive a booster dose of Comirnaty. The effectiveness of Comirnaty, even after a booster dose, may be lower in immunocompromised individuals. In these cases, you should continue to maintain physical precautions to help prevent COVID-19. Additionally, your close contacts should be vaccinated as appropriate. Discuss individual recommendations with your doctor.
Children
Comirnaty 30 micrograms/dose dispersion for injection is not recommended for use in children under 12 years of age.
A pediatric presentation is available for infants and children from 6 months to 4 years of age. For more information, see the package leaflet for Comirnaty 3 micrograms/dose concentrate for dispersion for injection.
A pediatric presentation is available for children from 5 to 11 years of age (i.e., from 5 to less than 12 years of age). For more information, see the package leaflet for Comirnaty 10 micrograms/dose concentrate for dispersion for injection.
Comirnaty is not recommended for use in infants under 6 months of age.
Other medicines and Comirnaty
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine or have recently received any other vaccine.
Pregnancy and breastfeeding
If you are pregnant or think you may be pregnant, tell your doctor, nurse, or pharmacist before receiving this vaccine.
Comirnaty can be used during pregnancy. A large amount of information on pregnant women vaccinated with Comirnaty during the second and third trimesters has not shown negative effects on pregnancy or on the newborn. Although information on the effects on pregnancy or the newborn after vaccination during the first trimester is limited, no change in the risk of spontaneous abortion has been observed.
Comirnaty can be used during breastfeeding.
Driving and using machines
Some of the effects of vaccination mentioned in section 4 (Possible side effects) may temporarily affect your ability to drive or use machines. Wait until these effects have disappeared before driving or using machines.
3. How Comirnaty is administered
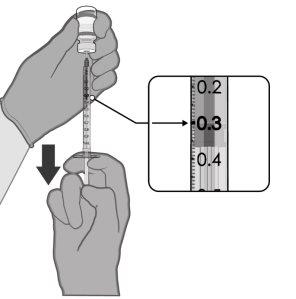
Comirnaty is administered as an injection of 0.3 ml into a muscle of the arm. You will receive two injections.
A second dose of the same vaccine is recommended 3 weeks after the first dose to complete the vaccination schedule.
If you are immunocompromised, you may receive a third dose of Comirnaty at least 28 days after the second dose.
A booster dose of Comirnaty can be administered at least 3 months after the most recent dose of a COVID-19 vaccine in individuals 12 years of age and older.
Consult your healthcare professional about eligibility for the booster dose and the timing of administration.
If you have any further questions on the use of Comirnaty, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all vaccines, Comirnaty can cause side effects, although not everybody gets them.
Very common side effects:may affect more than 1 in 10 people
- injection site: pain, swelling
- fatigue
- headache
- muscle pain
- chills
- joint pain
- diarrhea
- fever
Some of these side effects were slightly more frequent in adolescents from 12 to 15 years of age than in adults.
Common side effects:may affect up to 1 in 10 people
- redness at the injection site
- nausea
- vomiting
Uncommon side effects:may affect up to 1 in 100 people
- enlarged lymph nodes (more frequently observed after the booster dose)
- malaise
- arm pain
- insomnia
- itching at the injection site
- allergic reactions such as skin rash or itching
- feeling weak or lack of energy/drowsiness
- decreased appetite
- dizziness
- excessive sweating
- night sweats
Rare side effects:may affect up to 1 in 1,000 people
- temporary paralysis of one side of the face
- allergic reactions such as hives or swelling of the face
Very rare side effects:may affect up to 1 in 10,000 people
- inflammation of the heart muscle (myocarditis) or inflammation of the outer lining of the heart (pericarditis) that can lead to difficulty breathing, palpitations, or chest pain
Frequency not known(cannot be estimated from the available data)
- severe allergic reaction
- extensive swelling in the limb where the vaccine was administered
- swelling of the face (may occur in patients who have received dermal filler injections)
- a skin reaction that causes red spots or patches on the skin, which may look like a target or a "bull's eye" with a dark red center surrounded by lighter red rings (erythema multiforme)
- abnormal sensation in the skin, such as tingling or numbness (paresthesia)
- decreased sensitivity, especially in the skin (hypoesthesia)
- heavy menstrual bleeding (most cases do not appear to be serious and are temporary)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly via the national reporting system listed in Appendix V and include the batch number if available. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Comirnaty
Keep this medicine out of the sight and reach of children.
The following information on storage, expiry, and use and handling is intended for healthcare professionals.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date is the last day of the month stated.
Store in a freezer at –90 °C to –60 °C.
Store in the original packaging to protect from light.
The vaccine will be received frozen at –90 °C to –60 °C. The frozen vaccine can be stored at –90 °C to –60 °C or at 2 °C to 8 °C after receipt.
Single-dose vials: If stored frozen at –90 °C to –60 °C, the packs of 10 single-dose vials of the vaccine can be thawed at 2 °C to 8 °C for 2 hours or individual vials can be thawed at room temperature (up to 30 °C) for 30 minutes.
Multidose vials: If stored frozen at –90 °C to –60 °C, the packs of 10 multidose vials of the vaccine can be thawed at 2 °C to 8 °C for 6 hours or individual vials can be thawed at room temperature (up to 30 °C) for 30 minutes.
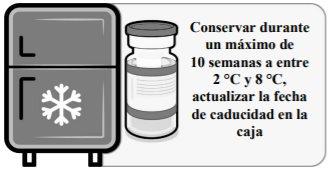
Thawed vials: Once removed from the freezer, the unopened vial can be stored and transported refrigerated at 2 °C to 8 °C for a maximum of 10 weeks; do not exceed the expiry date printed (EXP). The outer packaging should be marked with the new discard date at 2 °C to 8 °C. Once thawed, the vaccine cannot be re-frozen.
Before use, unopened vials can be stored for a maximum of 12 hours at temperatures between 8 °C and 30 °C.
Thawed vials can be handled in ambient light conditions.
Opened vials: After the first puncture, store the vaccine at 2 °C to 30 °C and use within 12 hours, including a transport time of up to 6 hours. Discard unused vaccine.
Do not use this vaccine if you notice visible particles or a change in color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Comirnaty Composition
- The active ingredient is a COVID-19 mRNA vaccine called tozinameran.
- A single-dose vial contains 1 dose of 0.3 ml with 30 micrograms of tozinameran each.
- A multi-dose vial contains 6 doses of 0.3 ml with 30 micrograms of tozinameran each.
- The other components are:
- ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) (ALC-0315)
- 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159)
- 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)
- cholesterol
- tromethamine
- tromethamine hydrochloride
- sucrose
- water for injectable preparations
Product Appearance and Container Contents
The vaccine is a dispersion (pH: 6.9-7.9) of a color between white and off-white that is presented in:
- a single-dose vial of 1 transparent dose (Type I glass), 2 ml, with a rubber stopper and a gray plastic flip-off cap with an aluminum seal; or
- a multi-dose vial of 6 doses, transparent (Type I glass), 2 ml, with a rubber stopper and a gray plastic flip-off cap with an aluminum seal.
Size of the single-dose vial container: 10 vials.
Sizes of the multi-dose vial container: 10 vials or 195 vials. Only some package sizes may be marketed.
Marketing Authorization Holder
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz
Germany
Phone: +49 6131 9084-0
Fax: +49 6131 9084-2121
Manufacturers
BioNTech Manufacturing GmbH
Kupferbergterrasse 17-19
55116 Mainz
Germany
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs
Belgium
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer S.A./N.V. Tel/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. +370 52 51 4000 |
| Magyarország Pfizer Kft Tel: +36 1 488 3700 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +35621 344610 |
Danmark Pfizer ApS Tlf: +45 44 201 100 | Norge Pfizer AS Tlf: +47 67 526 100 |
Deutschland BioNTech Manufacturing GmbH Tel: +49 6131 90840 | Nederland Pfizer BV Tel: +31 (0)10 406 43 01 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H Tel: +43 (0)1 521 15-0 |
Ελλάδα Pfizer Ελλάς A.E. Τηλ.: +30 210 6785 800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel:+ 34914909900 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél +33 1 58 07 34 40 | România Pfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel.: +386 (0) 1 52 11 400 |
Ireland Pfizer Healthcare Ireland Tel: 1800 633 363 (toll free) +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: +421 2 3355 5500 |
Ísland Icepharma hf Simi: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κύπρος Pfizer Ελλάς Α.Ε. (Cyprus Branch) Τηλ: +357 22 817690 | United Kingdom (Northern Ireland) Pfizer Limited Tel: +44 (0) 1304 616161 |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: +371 670 35 775 |
Date of Last Revision of this Leaflet:
Scan the code with a mobile device to obtain the leaflet in different languages.

URL: www.comirnatyglobal.com
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
The leaflet can be found in all languages of the European Union/European Economic Area on the European Medicines Agency website.
This information is intended for healthcare professionals only:
Administer Comirnaty intramuscularly in a primary series of 2 doses (0.3 ml each) with a separation of 3 weeks.
A third dose may be administered at least 28 days after the second dose in individuals who are severely immunocompromised.
A booster dose of Comirnaty (0.3 ml) may be administered at least 3 months after the most recent dose of a COVID-19 vaccine in individuals 12 years of age and older.
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product must be clearly recorded.
Handling Instructions
Comirnaty must be prepared by a healthcare professional using aseptic technique to ensure the sterility of the prepared dispersion.
INSTRUCTIONS APPLICABLE TO BOTH SINGLE-DOSE AND MULTI-DOSE VIALS VERIFICATION OF COMIRNATY 30 MICROGRAMS/DOSE DISPERSION FOR INJECTION (PERSONS 12 YEARS OF AGE AND OLDER) | |
|
|
HANDLING BEFORE USE OF COMIRNATY 30 MICROGRAMS/DOSE DISPERSION FOR INJECTION (PERSONS 12 YEARS OF AGE AND OLDER) | |
|
|
Gently 10 times |
|
PREPARATION OF INDIVIDUAL 0.3 ML DOSES OF COMIRNATY 30 MICROGRAMS/DOSE DISPERSION FOR INJECTION (PERSONS 12 YEARS OF AGE AND OLDER) | |
0.3 ml of vaccine | Single-dose vials
Multi-dose vials
To draw up 6 doses from the same vial, use syringes and/or needles with a low dead volume. The combination of syringe and needle with a low dead volume should have a dead volume of 35 microliters or less. If conventional syringes and needles are used, there may not be sufficient volume to draw up a sixth dose from the same vial.
|
Disposal
Disposal of unused medicinal products and all materials that have come into contact with them will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COMIRNATY 30 micrograms/dose Injectable DispersionDosage form: INJECTABLE, 0.1 mg/mlActive substance: covid-19, RNA-based vaccineManufacturer: Biontech Manufacturing GmbhPrescription requiredDosage form: INJECTABLE, 0.2 mLActive substance: covid-19, RNA-based vaccineManufacturer: Biontech Manufacturing GmbhPrescription requiredDosage form: INJECTABLE, 10 microgramsActive substance: covid-19, RNA-based vaccineManufacturer: Biontech Manufacturing GmbhPrescription required
Online doctors for COMIRNATY 30 micrograms/dose Injectable Dispersion
Discuss questions about COMIRNATY 30 micrograms/dose Injectable Dispersion, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions