
Cometriq 20mg hard capsules

How to use Cometriq 20mg hard capsules
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
COMETRIQ 20 mg hard capsules
COMETRIQ 80 mg hard capsules
(S)-cabozantinib malate
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is COMETRIQ and what is it used for
- What you need to know before you take COMETRIQ
- How to take COMETRIQ
- Possible side effects
- Storage of COMETRIQ
- Contents of the pack and other information
1. What is COMETRIQ and what is it used for
What is COMETRIQ
COMETRIQ is a cancer medicine that contains the active substance (S)-cabozantinib malate.
It is a medicine used to treat medullary thyroid cancer, a rare type of thyroid cancer that cannot be removed by surgery or that has spread to other parts of the body.
How does COMETRIQ work?
COMETRIQ blocks the action of proteins called tyrosine kinase receptors (RTKs), which are involved in cell growth and the development of new blood vessels that supply blood to these cells. These proteins may be present in high amounts in cancer cells, and by blocking their action, COMETRIQ can slow down the rate at which the tumor grows and help interrupt the blood supply that the cancer needs.
COMETRIQ may slow down or stop the growth of medullary thyroid cancer. It may help reduce the size of tumors associated with this type of cancer.
2. What you need to know before you take COMETRIQ
Do not take COMETRIQ
- if you are allergic to cabozantinib or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before starting COMETRIQ:
- If you have high blood pressure.
- If you have had or have an aneurysm (enlargement and weakening of the wall of a blood vessel) or a tear in the wall of a blood vessel.
- If you have diarrhea.
- If you have recently had coughing up blood or major bleeding.
- If you have had surgery in the last month (or if you have any scheduled), including dental procedures.
- If you have had radiation therapy in the last three months.
- If you have inflammatory bowel disease (such as Crohn's disease, ulcerative colitis, or diverticulitis).
- If you have been told that your cancer has spread to the respiratory tract or esophagus.
- If you have recently had a blood clot in your leg, stroke, or heart attack.
- If you are taking medicines to control your heart rate, have a slow heart rate, have heart problems, or have problems with calcium, potassium, or magnesium levels in your blood.
- If you have severe liver or kidney disease.
Tell your doctor if you are affected by any of these conditions.You may need treatment, or your doctor may decide to change the dose of COMETRIQ or stop treatment altogether. Also, see section 4: "Possible side effects".
You should also inform your dentist that you are taking COMETRIQ. It is important that you pay special attention to your oral hygiene during treatment with COMETRIQ.
Children and adolescents
COMETRIQ is not recommended for use in children and adolescents. The effects of COMETRIQ in people under 18 years of age are not known.
Taking COMETRIQ with other medicines:
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription. This is because COMETRIQ may affect how other medicines work. Some medicines may also affect how COMETRIQ works. This may require your doctor to change the doses you take.
- Medicines for treating fungal infections (such as itraconazole, ketoconazole, and posaconazole)
- Medicines used to treat bacterial infections (antibiotics), such as erythromycin, clarithromycin, and rifampicin
- Medicines for allergies, such as fexofenadine
- Medicines for treating angina (chest pain due to inadequate blood supply to the heart), such as ranolazine
- Steroids used to reduce inflammation or treat various immune system diseases
- Medicines used to treat epilepsy or seizures, such as phenytoin, carbamazepine, and phenobarbital
- Herbal medicines containing St. John's Wort (Hypericum perforatum), which is sometimes used to treat depression or depression-related conditions, such as anxiety
- Anticoagulant medicines such as warfarin and dabigatran etexilate
- Medicines for treating high blood pressure or other heart diseases, such as aliskiren, ambrisentan, digoxin, talinolol, and tolvaptan
- Medicines for diabetes, such as saxagliptin and sitagliptin
- Medicines for treating gout, such as colchicine
- Medicines used to treat HIV or AIDS, such as ritonavir, maraviroc, and emtricitabine
- Medicines used to treat viral infections, such as efavirenz
- Medicines used to prevent rejection after a transplant (cyclosporin) and treatments with cyclosporin in rheumatoid arthritis and psoriasis
Oral contraceptives
If you take COMETRIQ while using oral contraceptives, oral contraception may be ineffective. You should also use a barrier method (e.g., condom or diaphragm) while taking COMETRIQ and for at least 4 months after stopping treatment.
Taking COMETRIQ with food
Avoid taking products that contain grapefruit juice during the entire time you use this medicine, as they may increase the levels of COMETRIQ in your blood.
Pregnancy, breastfeeding, and fertility
Pregnancy should be avoided during treatment with COMETRIQ.If you or your partner may become pregnant, you must use adequate contraception during treatment and for at least 4 months after stopping treatment. Talk to your doctor about which contraceptive methods are appropriate while taking COMETRIQ. See section 2.
Tell your doctor if you or your partner become pregnant, or if you or your partner plan to become pregnant, during treatment with COMETRIQ.
Talk to your doctorbeforestarting COMETRIQif you or your partner are planning to have a child after treatment. There is a possibility that treatment with COMETRIQ may affect your fertility.
Women taking COMETRIQ should stop breastfeeding during treatment and for at least 4 months after treatment has stopped, as cabozantinib and/or its metabolites may be excreted in breast milk and be harmful to the baby.
Driving and using machines
Be cautious when driving or using machines. Keep in mind that treatment with COMETRIQ can make you feel tired or weak.
3. How to take COMETRIQ
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
Keep taking this medicine until your doctor decides to stop treatment. If you experience severe side effects, your doctor may decide to change the dose or stop treatment earlier than planned. Your doctor will decide if you need to adjust the dose, especially during the first 8 weeks of treatment with COMETRIQ.
You should take COMETRIQ once a day. Depending on the dose prescribed by your doctor, the number of capsules you should take will be as follows:
- 140 mg (one 80 mg orange capsule and three 20 mg grey capsules)
- 100 mg (one 80 mg orange capsule and one 20 mg grey capsule)
- 60 mg (three 20 mg grey capsules)
Your doctor will decide which dose is suitable for you.
The capsules are presented in blister strips organized by prescribed dose. Each blister strip has enough capsules to last 7 days (one week). The capsules are also presented in a 28-day pack that contains enough capsules for 28 days in 4 blister strips with 7 days' worth of capsules in each strip.
Each day, take all the capsules from one row. In section 6, later on, you will find more information about the blister strips, how many capsules you should take, and the total number of capsules each strip contains. To help you remember the doses, write the date you take your first dose in the space provided next to the capsules. To remove the capsules for the dose:

- Press the tab.
- Remove the paper from the back.
- Push the capsule through the aluminum foil.
Do nottake COMETRIQ with food. Do not eat any food for at least 2 hours before taking COMETRIQ and for 1 hour after taking it. Swallow the capsules one at a time with water. Do not open the capsules.
If you take more COMETRIQ than you should
If you have taken more COMETRIQ than prescribed, talk to a doctor or go to the hospital immediately with the capsules and this leaflet.
If you forget to take COMETRIQ
- If there are 12 or more hours until the next dose, take the missed dose immediately. Take the next dose at the usual time.
- If there are less than 12 hours until the next dose, do not take the missed dose. Take the next dose at the usual time.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you experience side effects, your doctor may tell you to take COMETRIQ at a lower dose. Your doctor may also prescribe you other medicines to help control side effects.
Tell your doctor immediately if you notice any of the following side effects, as you may need urgent medical treatment:
- Symptoms such as abdominal pain, nausea, vomiting, constipation, or fever. These symptoms can be a result of a gastrointestinal perforation, a hole in the stomach or intestine that can be life-threatening.
- Swelling, pain in hands and feet, or shortness of breath.
- A wound that does not heal.
- Vomiting or coughing up blood, which can be bright red or look like coffee grounds.
- Pain in the mouth, teeth, and/or jaw, swelling or sores in the mouth, numbness or a feeling of heaviness in the jaw or movement of a tooth. These could be signs of jawbone damage (osteonecrosis).
- Seizures, headache, confusion, or difficulty concentrating. These can be signs of a condition called posterior reversible encephalopathy syndrome (PRES). PRES is rare (affects less than 1 in 100 people).
- Diarrhea that is severe and does not seem to be getting better.
Other side effects include:
Very common side effects(may affect more than 1 in 10 people):
- Stomach problems, such as diarrhea, nausea, vomiting, constipation, indigestion, and abdominal pain
- Difficulty swallowing
- Blisters, pain in hands or soles of feet, skin rash or redness, dry skin
- Decreased appetite, weight loss, altered sense of taste
- Fatigue, weakness, headache, dizziness
- Changes in hair color (lightening), hair loss
- Hypertension (high blood pressure)
- Redness, swelling, or pain in the mouth or throat, difficulty speaking, hoarseness
- Changes in blood test results used to monitor general health and liver function, low levels of electrolytes (such as magnesium, calcium, or potassium)
- Low platelet count
- Pain in joints, muscle spasms
- Inflammation of lymph nodes
- Pain in arms, hands, legs, or feet
Common side effects(may affect up to 1 in 10 people):
- Anxiety, depression, confusion
- Generalized pain, chest pain or muscle pain, ear pain, ringing in the ears
- Weakness or decreased sensation or tingling in the limbs
- Chills, tremors
- Dehydration
- Inflammation of the abdomen or pancreas
- Inflammation of the lips and corners of the mouth
- Inflammation of the hair roots, acne, blisters (on parts of the body other than hands and feet)
- Swelling of the face and other parts of the body
- Loss or change of sense of taste
- Hypotension (low blood pressure)
- Atrial fibrillation (rapid and irregular heartbeat)
- Lightening of skin color, scaly skin, unusual paleness
- Abnormal hair growth
- Hemorrhoids
- Pneumonia (lung infection)
- Pain in the mouth, teeth, and/or jaw, swelling or sores in the mouth, numbness or a feeling of heaviness in the jaw or movement of a tooth
- Reduced thyroid activity, whose symptoms may include, among others: fatigue, weight gain, constipation, feeling cold, and dry skin
- Low white blood cell count
- Decrease in phosphate levels in the blood
- Tear, perforation, or bleeding in the stomach or intestine, inflammation or tear of the anus, bleeding in the lungs or trachea (airways)
- An abnormal connection of tissue in the digestive system, whose symptoms may include severe or persistent stomach pain
- An abnormal connection of tissue in the trachea (airways), esophagus, or lungs
- Abscess (collection of pus with swelling and inflammation) in the abdominal or pelvic area, or in the gums or teeth
- Blood clots in blood vessels and lungs
- Stroke
- Fungal infections (from fungi) in the skin, mouth, or genitals
- Wounds that have difficulty healing
- Presence of protein or blood in the urine, gallstones, pain when urinating
- Blurred vision
- Increased bilirubin levels in the blood (which can cause jaundice/yellow color in the skin or eyes)
- Decreased protein levels in the blood (albumin)
Uncommon side effects(may affect up to 1 in 100 people):
- Inflammation of the esophagus, whose symptoms may include, among others: chest pain, nausea, altered sense of taste, abdominal swelling, belching, and indigestion.
- Infection and inflammation of the lung, lung collapse
- Skin ulcers, cysts, red spots on the face or thighs
- Facial pain
- Changes in test results that measure blood clotting or blood cell count
- Muscle coordination loss, skeletal muscle injuries
- Loss of muscle coordination, loss of consciousness, speech disturbances, delirium, abnormal dreams
- Chest pain due to blockage in the arteries, palpitations
- Liver damage, kidney failure
- Hearing loss
- Eye inflammation, cataracts
- Interruption of menstruation, vaginal bleeding
- A condition called posterior reversible encephalopathy syndrome (PRES), whose symptoms include, among others: seizures, headaches, confusion, or difficulty concentrating
Not known(side effects with unknown frequency)
- Heart attack
- Enlargement and weakening of the wall of a blood vessel or tear in the wall of a blood vessel (aneurysms and arterial dissections)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of COMETRIQ
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister strip after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C. Store in the original package to protect from moisture.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
COMETRIQ composition
The active ingredient is (S)-cabozantinib malate
The hard capsules of COMETRIQ 20 mg contain (S)-cabozantinib malate, equivalent to 20 mg of cabozantinib.
The hard capsules of COMETRIQ 80 mg contain (S)-cabozantinib malate, equivalent to 80 mg of cabozantinib.
The other components are:
- Capsule content:microcrystalline cellulose, sodium croscarmellose, sodium starch glycolate, anhydrous colloidal silica, and stearic acid.
- Capsule shell:gelatin and titanium dioxide (E171).
- The 20 mg capsules also contain black iron oxide (E172).
- The 80 mg capsules also contain red iron oxide (E172).
- Printing ink:shellac lacquer, black iron oxide (E172), and propylene glycol.
Product appearance and container contents
COMETRIQ 20 mg hard capsules are gray capsules with "XL184 20mg" printed on one side.
COMETRIQ 80 mg hard capsules are orange capsules with "XL184 80mg" printed on one side.
The hard capsules of COMETRIQ are presented in blister strips organized according to the prescribed dose. Each blister strip contains sufficient medication for seven days. Each row of the strip contains a daily dose.
The blister strip with a daily dose of 60 mg contains twenty-one 20 mg capsules organized into a total of seven daily doses. Each daily dose occupies a row, which contains three 20 mg capsules:

The blister strip with a daily dose of 100 mg contains seven 80 mg capsules and seven 20 mg capsules, organized into a total of seven daily doses. Each daily dose occupies a row that contains one 80 mg capsule and one 20 mg capsule.
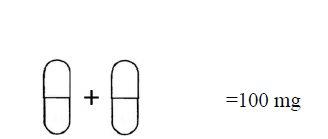
One orange 80 mg capsule + one gray 20 mg capsule.
The blister strip with a daily dose of 140 mg contains seven 80 mg capsules and twenty-one 20 mg capsules, organized into a total of seven doses. Each daily dose occupies a row that contains one 80 mg capsule and three 20 mg capsules:
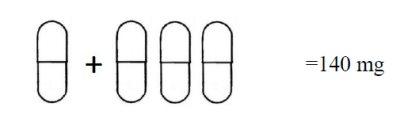
One orange 80 mg capsule + three gray 20 mg capsules.
COMETRIQ hard capsules are also presented in 28-day packs:
84 capsules (4 blister strips of 21 capsules of 20 mg) (60 mg/day dose)
56 capsules (4 blister strips of 7 capsules of 20 mg and 7 capsules of 80 mg) (100 mg/day dose)
112 capsules (4 blister strips of 21 capsules of 20 mg and 7 capsules of 80 mg) (140 mg/day dose)
Each 28-day pack contains sufficient medication for 28 days.
Marketing authorization holder
Ipsen Pharma
65 quai Georges Gorse
92100 Boulogne-Billancourt
France
Manufacturer
Catalent UK Packaging Limited
Lancaster Way
Wingates Industrial Park
Westhoughton
Bolton
Lancashire
BL5 3XX
United Kingdom
Or
Catalent Germany Schorndorf GmbH
Steinbeisstr. 1 und 2
73614 Schorndorf
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder.
Belgium/Luxembourg Ipsen NV Guldensporenpark 87 B-9820 Merelbeke Belgium Tel: + 32 - 9 - 243 96 00 | Italy Ipsen SpA Via del Bosco Rinnovato n. 6 Milanofiori Nord Palazzo U7 20090 Assago (Mi) Tel: + 39 - 02 - 39 22 41 |
Bulgaria PharmaSwiss EOOD 16, Troyanski Prohod Street, Floor 3, Office 8, Lagera 1612 Sofia Tel: +359 2 8952 110 | Latvia Ipsen Pharma representative office Kalnciema street 33-5 Riga LV 1046 Tel: +371 67622233 |
Czech Republic Ipsen Pharma, s.r.o. Olbrachtova 2006/9, 140 00 Praha 4 Tel: + 420 242 481 821 | Lithuania Ipsen Pharma SAS Lietuvos filialas
08105 Vilnius Tel. + 370 700 33305 |
Denmark, Norway, Finland, Sweden, Iceland Institut Produits Synthèse (IPSEN) AB Kista Science Tower Färögatan 33 SE- 164 51 Kista Sweden Tel: +46 8 451 60 00 | Hungary Ipsen Pharma Hungary Kft. Váci út 33. IX. em. H- 1134 Budapest Tel.: +36-1-555-5930 |
Germany, Austria Ipsen Pharma GmbH Einsteinstraße 174 D-81677 München Tel.: +49 89 2620 432 89 | Netherlands Ipsen Farmaceutica B.V. Taurusavenue 33b 2132 LS Hoofddorp Tel: + 31 (0) 23 554 1600 |
Estonia Centralpharma Communications OÜ Selise 26-11, 13522, Tallinn Tel: +372 60 15 540 | Poland Ipsen Poland Sp. z o.o. Al. Jana Pawla II 29 00-867 Warszawa Tel.: + 48 (0) 22 653 68 00 |
Greece, Cyprus, Malta Ipsen Μονοπρ?σωπη EΠΕ Αγ. Δημητρ?ου 63 ?λιμος GR-17456 Αθ?να Ελλ?δα Tel: + 30 - 210 - 984 3324 | Portugal Ipsen Portugal - Produtos Farmacêuticos S.A. Alameda Fernão Lopes, n° 16A-1°B Miraflores P-1495 - 190 Algés Portugal Tel: + 351 - 21 - 412 3550 |
Spain Ipsen Pharma, S.A. Torre Realia, Plaza de Europa, 41-43 08908 L?Hospitalet de Llobregat Barcelona Tel: + 34 - 936 - 858 100 | Romania Ipsen Pharma România SRL Sectorul 1, Strada Grigore Alexandrescu nr. 59, Etaj 1 Bucuresti, 010623 Tel: + 40 21 231 27 20 |
France, Croatia, Slovenia Ipsen Pharma 65 quai Georges Gorse 92100 Boulogne-Billancourt France Tél: + 33 1 58 33 50 00 | Slovenia PharmaSwiss d.o.o. Brodišce 32 SI-1236 Trzin Tel: + 386 1 236 47 00 Tel: + 44 (0)1753 - 62 77 00 |
Croatia PharmaSwiss d.o.o. Strojarska 20, 10 000 Zagreb Croatia Tel: +385 1 6311 833 Fax: +385 1 6311 844 | Slovak Republic Ipsen Pharma, organizacná zložka Zámocká 3 SK-811 01 Bratislava Slovak Republic Tel: + 420 242 481 821 |
Ireland Ipsen Pharmaceuticals Ltd. Blanchardstown Industrial Park Blanchardstown IRL-Dublin 15 Tel: +353-1-809-8256 | United Kingdom Ipsen Ltd. 190 Bath Road Slough, Berkshire SL1 3XE United Kingdom Tel: + 44 (0)1753 - 62 77 00 |
Date of last revision of this leaflet:
This medicinal product has been authorized with a "conditional approval".
This type of approval means that more information is expected to be obtained for this medicinal product.
The European Medicines Agency will review the new information for this medicinal product at least once a year, and this leaflet will be updated as necessary.
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Cometriq 20mg hard capsulesDosage form: TABLET, 20 mgActive substance: cabozantinibManufacturer: Ipsen PharmaPrescription requiredDosage form: TABLET, 40 mgActive substance: cabozantinibManufacturer: Ipsen PharmaPrescription requiredDosage form: TABLET, 60 mgActive substance: cabozantinibManufacturer: Ipsen PharmaPrescription required
Online doctors for Cometriq 20mg hard capsules
Discuss questions about Cometriq 20mg hard capsules, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






