COMBINEST PF 50 micrograms/ml + 5 mg/ml EYE DROPS SOLUTION


How to use COMBINEST PF 50 micrograms/ml + 5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Combinest PF 50 micrograms/ml + 5 mg/ml eye drops, solution
Latanoprost/timolol
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Combinest PF and what is it used for
- What you need to know before you use Combinest PF
- How to use Combinest PF
- Possible side effects
- Storage of Combinest PF
- Contents of the pack and other information
1. What is Combinest PF and what is it used for
This medicine contains two active substances: latanoprost and timolol. Latanoprost belongs to a group of medicines known as prostaglandin analogues. Timolol belongs to a group of medicines called beta-blockers. Latanoprost works by increasing the natural outflow of fluid from the eye into the bloodstream. Timolol works by reducing the production of fluid in the eye.
This medicine is used to reduce the pressure in the eye in case you have diseases known as open-angle glaucoma or ocular hypertension. Both of these diseases are related to an increase in pressure within the eye, which can eventually affect your vision. Your doctor will normally prescribe this medicine when other medicines have not worked adequately.
This medicine can be used in adult men and women (including the elderly), but it is not recommended for use in children and adolescents under 18 years of age.
This medicine is a sterile solution that does not contain preservatives.
2. What you need to know before you use Combinest PF
Do not use Combinest PF:
- If you are allergic to latanoprost or timolol, to beta-blockers or to any of the other ingredients of this medicine (listed in section 6).
- If you have or have had in the past respiratory problems such as asthma, severe chronic obstructive pulmonary disease (a serious lung disease that can cause wheezing, difficulty breathing and/or long-term coughing).
- If you have severe heart problems or heart rate disorders.
Warnings and precautions
Talk to your doctor or pharmacist before you start using this medicine, if you have or have had in the past:
- Coronary heart disease (symptoms may include chest pain or tightness, shortness of breath or difficulty breathing), heart failure, low blood pressure.
- Abnormal heart rhythms such as a slow heart rate.
- Breathing problems, asthma or chronic obstructive pulmonary disease.
- Diseases characterized by poor blood circulation (such as Raynaud's disease or Raynaud's syndrome).
- Diabetes, as timolol may mask the signs and symptoms of low blood sugar levels.
- Overactivity of the thyroid gland (hyperthyroidism), as timolol may mask signs and symptoms.
- If you are going to undergo eye surgery (including cataract surgery) or have had any type of eye surgery.
- If you have eye problems (such as pain, irritation or inflammation in the eye or blurred vision).
- If you have dry eye.
- If you use contact lenses. You can continue to use this medicine, but you must follow the instructions that are included in section 3 for contact lens users.
- If you have angina (in particular a type known as Prinzmetal's angina).
- If you have severe allergic reactions that usually require hospital treatment.
- If you have had or are having a viral infection in the eye caused by the herpes simplex virus (HSV).
Tell your doctor that you are using this medicine before undergoing surgery, as timolol may affect the effects of some medicines used during anesthesia.
Children and adolescents
This medicine is not recommended for use in children and adolescents under 18 years of age.
Other medicines and Combinest PF
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines, including eye drops and medicines obtained without a prescription.
This medicine may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or plan to use medicines to lower blood pressure, heart medicines or medicines to treat diabetes.
In particular, consult your doctor or pharmacist if you are taking any of the following types of medicines:
- Prostaglandins, prostaglandin analogues or prostaglandin derivatives (used for contraction and relaxation of smooth muscle, dilation and constriction of blood vessels, blood pressure control, and modulation of inflammation).
- Beta-blockers (used to treat high blood pressure, angina, to achieve a regular and adequate heart rate, heart attack, anxiety, migraine, glaucoma, and overactivity of the thyroid gland).
- Epinephrine (used to treat life-threatening allergic reactions caused by insect bites or stings, foods, medicines, latex, and other causes).
- Medicines used to treat high blood pressure such as calcium channel blockers, guanethidine, antiarrhythmics, digitalis glycosides or parasympathomimetics.
- Quinidine (used to treat heart diseases and certain types of malaria).
- Antidepressants such as fluoxetine and paroxetine.
Using Combinest PF with food and drink
Normal meals, food and drink have no effect on when or how you should use this medicine.
Pregnancy, breast-feeding and fertility
Pregnancy
Do not use this medicine if you are pregnant unless your doctor considers it necessary. If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
Breast-feeding
Do not use this medicine if you are breast-feeding. This medicine may pass into breast milk. Ask your doctor for advice before taking any medicine during breast-feeding.
Fertility
In animal studies, latanoprost and timolol have not been found to have any effect on male or female fertility.
Driving and using machines
When using this medicine, you may experience blurred vision for a short period of time. If this happens, do not drive or use tools or machines until your vision is clear again.
Combinest PF containsmacrogolglycerol hydroxystearate 40
This medicine contains macrogolglycerol hydroxystearate 40, which may cause skin reactions.
Combinest PF containsphosphate buffer
This medicine contains 6.54 mg of phosphates in each ml of solution.
If you have severe damage to the clear layer on the front of the eye (cornea), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium deposits.
Use in athletes
This medicine contains timolol, which may produce a positive result in doping tests.
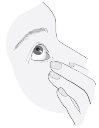
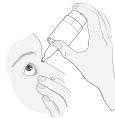
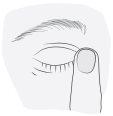
3. How to use Combinest PF
Follow the instructions for administration given by your doctor or pharmacist. Ask your doctor or pharmacist if you have any questions.
The recommended dose for adults (including elderly patients) is one drop once daily in the affected eye(s).
Do not use this medicine more than once a day, as the effectiveness of the treatment may decrease if it is administered more frequently.
Use this medicine as your doctor has told you. Continue using it until your doctor tells you to stop.
Your doctor may want to perform additional heart and circulatory tests if you are using this medicine.
Contact lens users
If you use contact lenses, you should remove them before using this medicine. After applying this medicine, wait 15 minutes before putting your contact lenses back in.
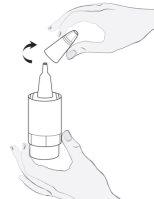
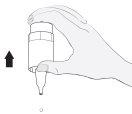
Instructions for use
1a
1b |
|
|
|
3 |
|
4 |
|
5 |
|
2.5 ml
5 ml
|
If you use Combinest PF with other eye drops
Wait at least 5 minutes between the application of this medicine and the administration of other eye drops.
If you use more Combinest PF than you should
If you have applied more drops in the eye than you should, you may feel a slight irritation in the eye and your eyes may also become red and watery. This should go away, but if you are concerned, contact your doctor.
If you swallow Combinest PF
In case of accidental ingestion of this medicine, consult your doctor or call the Toxicology Information Service, telephone: 91 562 04 20. If you swallow a large amount of this medicine, you may feel unwell, have stomach pain, feel tired, hot, dizzy and start sweating.
If you forget to use Combinest PF
Continue with the next dose as usual. Do not use a double dose to make up for the forgotten dose. If you have any doubts, consult your doctor or pharmacist.
If you have any doubts about the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
You can continue using the eye drops as usual, unless the adverse effects are severe. If you are concerned, consult your doctor or pharmacist. Do not stop using this medicine without consulting your doctor.
The following are known adverse effects associated with the use of eye drops containing the active substances latanoprost and timolol. The most important adverse effect is the possibility of a gradual and permanent change in eye color. It is also possible that eye drops containing the active substances latanoprost and timolol may cause serious changes in the way the heart works. If you notice any change in heart rate or cardiac function, you should consult your doctor and tell them that you have been using this medicine.
The following adverse effects have been observed with the use of eye drops containing the active substances latanoprost and timolol:
Very Common(may affect more than 1 in 10 people):
- Gradual change in eye color due to an increase in the amount of brown pigment in the colored part of the eye, known as the iris. If you have mixed-color eyes (blue-brown, gray-brown, yellow-brown, or green-brown), you are more likely to experience this change than if your eyes are a single color (blue, gray, green, or brown). The change in eye color may take years to develop. The change in eye color may be permanent and may be more noticeable if you use this medicine in only one eye. The change in eye color does not appear to be associated with the development of any problems. The change in eye color does not progress once treatment with this medicine has been discontinued.
Common(may affect up to 1 in 10 people):
- Eye irritation (feeling of stinging, feeling of grit in the eye, itching, pinching, and feeling of a foreign body in the eye) and eye pain.
Uncommon(may affect up to 1 in 100 people):
- Headache.
- Redness of the eyes, eye infection (conjunctivitis), blurred vision, tearing, eyelid inflammation, irritation or erosion of the eye surface.
- Rash or itching of the skin (pruritus).
- Nausea.
- Vomiting.
Other Adverse Effects
Like other medicines used in the eyes, latanoprost and timolol are absorbed into the bloodstream. The incidence of adverse effects after using eye drops is lower than when medicines are taken orally or injected.
Although not seen with eye drops containing both active substances latanoprost and timolol, the following adverse effects have been observed with one of the components of this medicine and may therefore occur with the use of this medicine. The adverse effects listed include reactions observed within the group of beta-blockers (e.g., timolol) when used to treat eye conditions:
- Development of a viral eye infection caused by the herpes simplex virus (HSV).
- Generalized allergic reactions, including swelling under the skin that can occur in areas such as the face and limbs and can obstruct the airway, causing difficulty swallowing or breathing, urticaria or itchy rash, localized or generalized rash, itching, sudden, severe, and potentially life-threatening allergic reaction.
- Low blood sugar levels.
- Dizziness.
- Difficulty sleeping (insomnia), depression, nightmares, memory loss, hallucination.
- Fainting, stroke, insufficient blood supply to the brain, worsening of myasthenia gravis (muscle disorder), unusual sensation like tingling and headache.
- Swelling of the back of the eye (macular edema), fluid-filled cyst in the colored part of the eye (iris cyst), sensitivity to light (photophobia), appearance of sunken eyes (increased depth of the eyelid sulcus).
- Signs and symptoms of eye irritation (e.g., burning, stinging, itching, tearing, redness), eyelid inflammation, corneal inflammation, blurred vision, and detachment of the layer under the retina after filtration surgery, which can cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the outer layer of the eyeball), drooping of the upper eyelid (causing the eye to be half-closed), double vision.
- Darkening of the skin around the eyes, changes in eyelashes and fine hair around the eye (increased number, length, thickness, and darkening), changes in the direction of eyelash growth, swelling around the eye, swelling of the colored part of the eye (iritis/uveitis), scarring of the eye surface.
- Ringing/buzzing in the ears (tinnitus).
- Angina, worsening of angina in patients who already had heart disease.
- Low heart rate, chest pain, palpitations (feeling the heartbeat), edema (fluid accumulation), changes in heart rate or rhythm, congestive heart failure (heart disease with difficulty breathing and swelling of feet and legs due to fluid accumulation), a type of heart rhythm disorder, heart attack, heart failure.
- Low blood pressure, poor blood circulation that causes fingers and toes to become numb and pale, cold hands and feet.
- Difficulty breathing, constriction of airways in the lungs (mainly in patients with pre-existing disease), difficulty breathing, cough, asthma, worsening of asthma.
- Altered taste, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting.
- Hair loss, skin rash with a silvery-white appearance (psoriasiform rash) or worsening of psoriasis, skin rash.
- Joint pain, muscle pain not caused by exercise, muscle weakness, fatigue.
- Sexual dysfunction, decreased libido.
In very rare cases, some patients with severe damage to the front transparent part of the eye (cornea) have developed cloudy areas in the cornea due to calcium deposits produced during treatment.
Reporting Adverse Effects:
If you experience any adverse effects, consult your doctor, even if they are possible adverse effects that are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Combinest PF
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and bottle after CAD. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
2.5 ml
You must discard each bottle after 4 weeks of first opening, to prevent infections. Write the date of opening on the carton in the space provided.
5 ml
You must discard each bottle after 8 weeks of first opening, to prevent infections. Write the date of opening on the carton in the space provided.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Combinest PF Composition
- The active substances are latanoprost and timolol.
Each ml of solution contains 50 micrograms of latanoprost and 5 mg of timolol (as 6.8 mg of timolol maleate).
- The other ingredients are: macrogolglycerol hydroxystearate 40, sodium chloride, disodium edetate, sodium dihydrogen phosphate dihydrate, disodium phosphate, hydrochloric acid and/or sodium hydroxide (for pH adjustment), water for injections
Appearance and Package Contents
2.5 ml
Combinest PF eye drops solution is a clear, colorless, and particle-free aqueous solution of 2.5 ml.
It is marketed in a carton, which contains a 5 ml multidose bottle (HDPE) with a pump (PP, HDPE, LDPE) and a green or orange pressure cylinder and cap (HDPE).
5 ml
Combinest PF eye drops solution is a clear, colorless, and particle-free aqueous solution of 5 ml.
It is marketed in a carton, which contains a 5 ml multidose bottle (HDPE) with a pump (PP, HDPE, LDPE) and a green or orange pressure cylinder and cap (HDPE).
Package sizes: Carton with 1 or 3 bottles of 2.5 ml solution or 1 or 3 bottles of 5 ml solution.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
Spain
Manufacturer
Lomapharm GmbH
Langes Feld 5
31860 Emmerthal
Germany
or
Pharmathen S.A.
Dervenakion Str. 6
Pallini 15351
Attiki
Greece
This medicine is authorized in the Member States of the European Economic Area under the following names:
Name | Country |
Latanoprost/Timolol Pharmathen | Denmark, France, Belgium, Germany, Austria, Slovenia |
Lonata | Cyprus, Greece |
Latanoprost e Timololo Pharmathen | Italy |
COMBINEST PF | Spain |
Latanotim | Netherlands |
Date of Last Revision of this Leaflet:March 2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COMBINEST PF 50 micrograms/ml + 5 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for COMBINEST PF 50 micrograms/ml + 5 mg/ml EYE DROPS SOLUTION
Discuss questions about COMBINEST PF 50 micrograms/ml + 5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




 2
2