
COLPOTROFIN 10 mg/g VAGINAL CREAM

How to use COLPOTROFIN 10 mg/g VAGINAL CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Colpotrofin 10 mg/g vaginal cream and what is it used for
- What you need to know before you start using Colpotrofin 10 mg/g vaginal cream
- How to use Colpotrofin 10 mg/g vaginal cream
- Possible Adverse Effects
- Conservation of Colpotrofin 10 mg/g Vaginal Cream
- Package Contents and Additional Information
Introduction
Package Leaflet: Information for the User
COLPOTROFÍN 10 mg/g Vaginal Cream
Promestriene
Read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Colpotrofin 10 mg/g vaginal cream and what is it used for
- What you need to know before you start using Colpotrofin 10 mg/g vaginal cream
- How to use Colpotrofin 10 mg/g vaginal cream
- Possible side effects
- Storing Colpotrofin 10 mg/g vaginal cream
- Contents of the pack and other information
1. What is Colpotrofin 10 mg/g vaginal cream and what is it used for
Colpotrofin belongs to the group of estrogen (female sex hormone) medicines.
Colpotrofin is used to treat atrophic disorders of the female genital area (vulva, vestibule, and vaginal ring) that occur in situations where there is a decrease in estrogens, such as menopause, castration, use of contraceptives, etc., and which manifest with dryness, itching, or irritation of the genital skin and mucosa.
2. What you need to know before you start using Colpotrofin 10 mg/g vaginal cream
Do not useColpotrofin
- if you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
- if you are breastfeeding.
- if you have a history, known presence, or suspicion of breast cancer.
- if you have a history or suspicion of malignant tumors dependent on estrogens (e.g., endometrial cancer).
- if you have undiagnosed genital bleeding.
- if you have untreated endometrial hyperplasia.
- if you have had venous thromboembolism (deep vein thrombosis, pulmonary embolism)
- if you have known thrombophilic disorders (e.g., protein C, protein S, or antithrombin deficiency, see "Warnings and precautions").
- if you have had recent or active arterial thromboembolic disease (e.g., angina pectoris or myocardial infarction)
- if you have acute liver disease or a history of liver disease when liver function values have not returned to normal
- if you have porphyria (a rare, usually inherited disorder in which large amounts of porphyrin are excreted in feces and urine).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colpotrofin. Before initiating or resuming treatment, a complete personal and family medical history should be taken, and periodic checks should be adapted individually to each woman, including mammograms. Changes in the breasts should be communicated to your doctor.
Be especially careful with Colpotrofin:
- if you have or have had vaginal stenosis (narrowing of the vagina) or vaginal prolapse.
- if you have or have had a disease characterized by the growth of tissue that lines the uterus outside of it (endometriosis) or a type of benign tumor of the uterus (uterine fibroid)
For the treatment of postmenopausal symptoms, local estrogen therapy should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful assessment of the risks and benefits should be made at least annually, and therapy should only be continued if the benefit outweighs the risk.
The data on the risks associated with hormone replacement therapy (HRT) in the treatment of early menopause are limited. However, due to the low absolute risk levels in young women, the balance of benefits and risks for young women may be more favorable than in older women.
Situations that require supervision
If any of the following situations affect you, have previously affected you, and/or have worsened during pregnancy or before hormonal treatment, inform your doctor immediately, as you will need to be closely monitored. It should be noted that these situations may recur or worsen during treatment with promestriene, in particular:
- Uterine fibroids or endometriosis
- Risk factors for thromboembolic disorders
- Risk factors for estrogen-dependent tumors, e.g., hereditary breast cancer
- High blood pressure
- Liver disorders (e.g., liver adenoma)
- Diabetes mellitus with or without vascular complications
- Gallstones (cholelithiasis)
- Migraine or severe headache
- Systemic lupus erythematosus (a disease of the immune system that affects connective tissue)
- History of endometrial hyperplasia
- Epilepsy
- Asthma
- Otosclerosis (a disease that affects the eardrum and hearing)
Reasons for immediate discontinuation of treatment
Treatment should be discontinued in case of discovery of a contraindication and in the following situations:
- Yellowing of the skin or mucous membranes (jaundice) or deterioration of liver function
- Significant increase in blood pressure.
- New onset of migraine-type headache
- Pregnancy
Endometrial hyperplasia and carcinoma
The long-term endometrial safety (more than one year) or repeated use of estrogens administered locally vaginally is uncertain. Therefore, if treatment is repeated, it should be reviewed at least annually.
If you have had a hysterectomy (removal of the uterus) due to endometriosis and it is known that you have residual endometriosis, inform your doctor, as caution is recommended when using this class of medications.
If bleeding or spotting occurs at any time during treatment, inform your doctor, as the reason should be investigated, and your doctor may perform an endometrial biopsy.
HRT and cancer
The following risks apply to hormone replacement therapy (HRT) medications that circulate in the blood. However, Colpotrofin is used for local treatment in the vagina, and absorption into the blood is very small. It is less likely that the disorders mentioned below will worsen or recur during treatment with Colpotrofin, but you should consult your doctor if you are concerned.
Breast cancer
Available data indicate that using Colpotrofin does not increase the risk of breast cancer in women who have not had breast cancer in the past. It is not known if Colpotrofin can be used safely in women who have had breast cancer in the past.
Ovarian cancer
Ovarian cancer is much rarer than breast cancer.
Epidemiological data suggest a slight increase in risk in women taking systemic hormone replacement therapy (HRT) with only estrogens, which becomes apparent within 5 years of use and decreases over time after treatment is stopped.
Venous thromboembolism
Systemic hormone replacement therapy (HRT) is associated with a 1.3-3 times higher risk of developing venous thromboembolism (VTE), i.e., deep vein thrombosis or pulmonary embolism. The occurrence of such an event is more likely in the first year of hormone replacement therapy (HRT) than later (see section 4).
Generally, patients with known thromboembolic conditions have a higher risk of VTE, and hormone replacement therapy (HRT) may increase this risk. Therefore, hormone replacement therapy (HRT) is contraindicated in these patients (see section 2 "Do not use Colpotrofin").
If you need to undergo prolonged immobilization after elective surgery, it is recommended to temporarily discontinue hormone replacement therapy (HRT) 4-6 weeks before. Treatment should not be resumed until you can move completely again.
Women without a personal history of VTE but with a close relative with a history of thrombosis at a young age should be carefully evaluated.
Hormone replacement therapy (HRT) is contraindicated if a thrombophilic defect is identified that has caused thrombosis in family members or if the defect is severe (e.g., antithrombin, protein C, or protein S deficiency or a combination of defects).
Women who are already receiving chronic treatment with anticoagulants require a careful assessment of the benefit-risk of using hormone replacement therapy (HRT).
If VTE occurs after starting treatment, the medication should be discontinued. You should contact your doctor immediately if you detect any potential thromboembolic symptoms (e.g., painful swelling of a leg, sudden chest pain, shortness of breath).
Arterial coronary disease (ACD)
Data found in studies did not increase the risk of ACD in hysterectomized women using systemic estrogen-only therapy.
Ischemic stroke
Systemic estrogen-only therapy is associated with a 1.5 times higher risk of ischemic stroke.
This relative risk is not dependent on age or duration of use, but as the base risk is strongly dependent on age, the overall risk of stroke in women using hormone replacement therapy (HRT) will increase with age, see section 4.
Other situations
Estrogens can cause fluid retention, and therefore, patients with heart or kidney dysfunction should be carefully monitored.
If you have pre-existing hypertriglyceridemia, your doctor will closely monitor you during estrogen replacement therapy or hormone replacement therapy (HRT), as rare cases of significant increases in plasma triglycerides leading to pancreatitis have been reported with estrogen therapy in this condition.
There is some evidence of an increased risk of probable dementia in women who start using combined continuous or estrogen-only hormone replacement therapy (HRT) after the age of 65.
Using Colpotrofin with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Due to the intravaginal administration of promestriene and its minimal systemic absorption, it is unlikely that any clinically significant drug interactions will occur with promestriene. However, interactions with other local treatments applied vaginally should be considered.
The use of this medicine with spermicides (substances that alter sperm motility or kill sperm) is not recommended. Local vaginal treatment could inactivate the contraceptive action of the spermicide.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
There is not enough information on the use of promestriene in pregnant women. Promestriene is not indicated during pregnancy. If you become pregnant during treatment with promestriene, you should discontinue it immediately.
The results of most current epidemiological studies on accidental fetal exposure to estrogens indicate that there are no teratogenic or fetotoxic effects.
Breastfeeding
The use of this medicine is not recommended during breastfeeding because the medicine may pass into breast milk.
Driving and using machines
Not applicable.
Colpotrofin contains methyl parahydroxybenzoate, sodium salt (E-219) and propyl parahydroxybenzoate, sodium salt (E-217)
This medicine may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate, sodium salt (E-219) and propyl parahydroxybenzoate, sodium salt (E-217).
3. How to use Colpotrofin 10 mg/g vaginal cream
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Your doctor will prescribe the lowest dose to treat your symptoms for the shortest possible time. Consult your doctor if you think the dose is too strong or too weak.
This medicine is administered vaginally.
The recommended dose is:
- One or two applications per day of the amount of cream necessary to cover the surface to be treated, followed by gentle massage.
- In cases where intravaginal application of the cream is considered convenient, it will be performed 1-2 times a day, with one of the doses preferably administered at night, before bedtime, equivalent to a dose of 10-20 mg of promestriene per day
The doctor may modify the dose based on the improvement observed.
Treatment should last an average of 3 weeks.
If the cause of the disorder persists (menopause, castration, contraceptive treatments with estrogens-progestogens) or if the iatrogenic effect (due to medical intervention) is lasting (irradiation), maintenance treatments may be necessary.
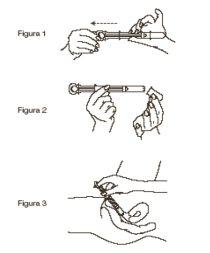
Intravaginal application method:
- For intravaginal application of the cream, the special applicator included in the package will be used, loading it directly from the tube, to which the applicator is adjusted, up to the signal that appears on the applicator and which corresponds to 1 gram of cream (Figure 1).
- Once loaded, it will be inserted into the vagina to the desired depth, injecting the dose by pressing the plunger (Figure 2).
- Application is simpler in a supine position with legs flexed and slightly separated (Figure 3).
After using the applicator, it should be washed with warm water for better preservation and subsequent use.
If you need to undergo surgery
If you are going to undergo surgery, inform the surgeon that you are using Colpotrofin. You may need to stop using Colpotrofin during 4-6 weeks before the operation to reduce the risk of a blood clot (see section 2). Ask your doctor when you can start using Colpotrofin again.
If you use more Colpotrofin than you should
Given the route of administration, it is unlikely that an overdose will occur.
However, excessive use may lead to the exacerbation of local side effects such as irritation, itching, and burning sensation in the vulvar area.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Colpotrofin
Do not use a double dose to make up for forgotten doses.
If you forgot to use this medicine when it was due, apply the cream as soon as you remember and continue with your usual treatment regimen.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
Very rare (may affect up to 1 in 10,000 people): allergic reactions (e.g., rash, eczema, severe allergic reaction).
Very rare (may affect up to 1 in 10,000 people): itching in the vagina/vulva
Frequency not known (frequency cannot be estimated from available data): Vaginal bleeding.
Frequency not known (frequency cannot be estimated from available data): burning sensation in the vagina/vulva, discomfort in the vagina/vulva, pain in the vagina/vulva, vaginal discharge.
Class Effects Associated with Systemic Hormone Replacement Therapy (HRT)
The following risks have been associated withsystemic hormone replacement therapy (HRT) and apply to a lesser extent to estrogen products for vaginal application, where systemic exposure to estrogens remains within the normal postmenopausal range.
The following diseases are more common in women using systemic HRT medications than in women who do not use HRT. These risks are less related to treatments administered via the vaginal route, such as Colpotrofin.
Ovarian Cancer
The use of systemic hormone replacement therapy (HRT) has been associated with a slight increase in the risk of being diagnosed with ovarian cancer (see section 2).
Risk of Venous Thromboembolism
Systemic hormone replacement therapy (HRT) has been associated with an increased risk of 1.3-3 times more of developing venous thromboembolism (VTE), i.e., deep vein thrombosis or pulmonary embolism.The incidence of such an event is more likely in the first year of using hormone therapy (see section 2).
Risk of Stroke
The use of systemic hormone replacement therapy (HRT) is associated with an increased risk of up to 1.5 times more of stroke.The risk of hemorrhagic stroke does not increase during the use of hormone replacement therapy (HRT).
This relative risk is not dependent on age or duration of use, but as the base risk is strongly dependent on age, the overall risk of stroke in women using hormone replacement therapy (HRT) will increase with age, see section 2.
Other adverse reactions have been reported in association with systemicestrogen/progestogen treatment: Risk estimates were based on systemic exposure to estrogens and it is not known how they can be extrapolated to local treatment:
- Gallbladder disease
- Disorders of the skin and subcutaneous tissue: yellowish-brown spots on the skin, particularly on the face (chloasma), reddish lesions on the extremities and inside the mouth (erythema multiforme), inflammation in the form of lumps under the skin (erythema nodosum), red or purple discoloration of the skin and/or mucous membranes (vascular purpura).
- Probable dementia above 65 years of age (see section 2).
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Colpotrofin 10 mg/g Vaginal Cream
This medicine does not require special storage conditions.
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the box after CAD. The expiration date is the last day of the month indicated.
Medicines should not be thrown away through wastewater or household waste. Deposit the containers and medicines you no longer need at the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Colpotrofin
- The active ingredient is promestriene. Each gram of cream contains 10 mg of promestriene.
- The other components (excipients) are glycerol monostearate, decyl oleate, medium-chain triglycerides, glycerol (E-422), eumulgin, methyl parahydroxybenzoate, sodium salt (E-219), propyl parahydroxybenzoate, sodium salt (E-217), and purified water.
Appearance of the Product and Package Contents
Colpotrofin is a white, greasy cream.
It is presented in an aluminum tube with a polypropylene screw cap and an applicator containing 15 g or 30 g of cream.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Theramex Ireland Limited
3rd Floor, Kilmore House,
Park Lane, Spencer Dock,
Dublin 1
D01 YE64
Ireland
Manufacturer
Laboratoires CHEMINEAU
93 route de Monnaie
37210 Vouvray
France
Local Representative
Theramex Healthcare Spain, S.L.
Calle Martínez Villergas 52, Edificio C, planta 2ª izquierda.
28027 Madrid
Spain
Date of the Last Revision of this Prospectus: March 2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price11.4 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLPOTROFIN 10 mg/g VAGINAL CREAMDosage form: VAGINAL SEMISOLID, 50 micrograms/gActive substance: estriolManufacturer: Italfarmaco S.A.Prescription requiredDosage form: GEL, 0.5 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: GEL, 1 mgActive substance: estradiolManufacturer: Orion CorporationPrescription required
Online doctors for COLPOTROFIN 10 mg/g VAGINAL CREAM
Discuss questions about COLPOTROFIN 10 mg/g VAGINAL CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











