
COLIROFTA ANESTHETIC 5 mg/ml + 0.5 mg/ml EYE DROPS SOLUTION

How to use COLIROFTA ANESTHETIC 5 mg/ml + 0.5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
COLIROFTA ANESTHETIC 5 mg/ml + 0.5 mg/ml eye drops solution
Tetracaine hydrochloride/Naphazoline hydrochloride
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What COLIROFTA ANESTHETIC is and what it is used for
- What you need to know before starting to use COLIROFTA ANESTHETIC
- How to use COLIROFTA ANESTHETIC
- Possible side effects
5 Conservation of COLIROFTA ANESTHETIC
- Package contents and additional information
1. What COLIROFTA ANESTHETIC is and what it is used for
It is an eye drop that contains two active ingredients, tetracaine, a local anesthetic (temporarily numbs the area of administration), and naphazoline with decongestant action (produces vasoconstriction or narrowing of the visible blood vessels in the eye), which prolongs the anesthetic activity.
Colirofta Anesthetic is indicated for the treatment of painful eye conditions, removal of foreign bodies, eye examination, tonometry (measurement of pressure inside the eyes), gonioscopy (measures the angle of the iris and cornea, whether it is wide or narrow), and fundus examination with contact lens.
2. What you need to know before starting to use COLIROFTA ANESTHETIC
Do not use Colirofta Anesthetic
- If you are allergic to naphazoline, tetracaine, or other local anesthetics of the same type, or to any of the other components of this medicine (listed in section 6).
- If you have narrow-angle glaucoma.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colirofta Anesthetic.
Use this medicine only in the eye(s).
You should be cautious if you are in any of the following situations:
- If you are being treated with monoamine oxidase inhibitors (MAOIs) (usually antidepressants) or during the two weeks after stopping such treatment.
- In case you are being administered certain anesthetics (e.g., halothane, which makes the heart more sensitive to medications of the naphazoline type).
- If you have bronchial asthma.
- If you have any heart or circulation disease.
- If you have cerebral arteriosclerosis.
- If you have hypertension.
- If you have hyperthyroidism (excessive functioning of the thyroid gland).
- If you have diabetes.
The repeated use of this medicine (due to tetracaine) may cause corneal ulcers.
With continued use of tetracaine, a decrease in the duration of anesthesia and delayed healing may occur, among other visual side effects.
- Due to the anesthetic effect, your eyes will lose sensitivity, and you should be careful to avoid accidental injuries to the eyes.
- This medicine should only be used under the direct supervision of a healthcare professional.
- Do not use for more than 3 to 5 days.
- Do not wear contact lenses during the use of this medicine.
Use in elderly patients
See section 3.
Caution should be exercised in patients over 65 years old, particularly those with severe heart or circulation diseases (such as arrhythmias or hypertension), as naphazoline may worsen these conditions.
Children
Do not use this medicine in children; the efficacy and safety have not been established in children.
Other medicines and COLIROFTA ANESTHETIC
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Especially consult your doctor if you are using:
- Monoamine oxidase inhibitors (MAOIs) (you may suffer a severe hypertensive crisis).
- Sulfonamides in eye drops or ointment for the eyes.
- St. John's Wort.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Colirofta Anesthetic during pregnancy.
The doctor should decide whether to interrupt breastfeeding or treatment after considering the benefit versus the possible risk.
Driving and using machines
You may notice that your vision becomes blurred or you experience other discomforts for a while after applying the eye drops. Do not drive or use machines until these effects have disappeared.
COLIROFTA ANESTHETIC contains phosphates
This medicine contains 5.8 mg of phosphates per ml.
If you have severe corneal damage (the transparent layer on the front of the eye), treatment with phosphates, in very rare cases, can cause blurred vision due to calcium accumulation.
3. How to use COLIROFTA ANESTHETIC
Follow the administration instructions for this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults
Generally, instill 1 or 2 drops into the affected eye as needed.
Use in elderly patients
It is recommended to start treatment with the lowest recommended dose, as these patients may have a variable response to one of the components of the medicine, tetracaine.
Use in case of hepatic and/or renal insufficiency
The safety and efficacy have not been established in patients with hepatic and/or renal insufficiency.
Use in children
The safety and efficacy of this medicine have not been established in children.
Recommendations for use:
Ophthalmic route (in the eyes).
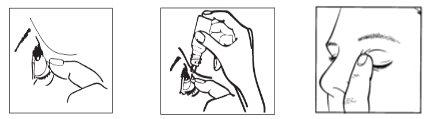
1 2 3
- Wash your hands.
- Take the bottle (dropper).
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back. Gently separate the eyelid from the eye with a finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or surrounding areas, with the dropper. The eye drops could become contaminated.
- Gently squeeze the base of the bottle to release one drop at a time (figure 2).
- After using this eye drop, press the edge of the eye next to the nose with your finger. This helps prevent the medicine from entering the rest of the body (figure 3).
- If you are applying drops to both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after using the medicine.
If a drop falls outsidethe eye, try again.
If you are using other ophthalmic medicines, wait at least 5 minutes between the administration of this eye drop and the other ophthalmic medicines. Ophthalmic ointments should be administered last.
If you use more COLIROFTA ANESTHETIC than you should
Taking a considerably larger dose of Colirofta Anesthetic than the recommended dose, for example, if you accidentally swallow Colirofta Anesthetic, may cause serious side effects that affect the heart and circulation. The symptoms may include: decreased heart rate (bradycardia), severe headache, nausea, vomiting, respiratory problems, increased heart rate (tachycardia), and chest pain.
An overdose in the eyes can be eliminated by rinsing the eyes with lukewarm water. Do not apply more drops until the next dose.
In case of severe overdose or accidental ingestion, consult your doctor or pharmacist immediately or the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
Severe reactions such as a drop in body temperature, drowsiness, or other symptoms of central nervous system depression (especially in children) and severe reactions that affect the cardiovascular system may occur.
After ingestion, especially by children, symptoms such as nausea, vomiting, lethargy, palpitations, difficulty breathing, among others, may appear.
If you forget to use COLIROFTA ANESTHETIC
Do not use a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember. However, if it is almost time for the next dose, continue with the next dose of your regular schedule.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
The following side effects have been reported, whose frequency is not known:
Due to the anesthetic, excessive use of this medicine may cause corneal ulcers or eye damage.
Rarely, allergic reactions may occur.
Due to the presence of naphazoline in the medicine, you may experience increased pupil size and increased pressure in the eye. General side effects such as headache, increased blood pressure, heart rate, and irregular heartbeat, increased heart rate, nausea, fainting, and stroke may occur.
Other side effects in children
Excessive use of naphazoline in infants and young children may cause central nervous system depression and a significant decrease in body temperature.
Rarely, itching has been observed after application.
Continuous redness and eye irritation, conjunctivitis, and eye pain have also been reported.
In predisposed patients, in general, and with greater use and frequency than recommended, tremors, weakness, and sweating may occur.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es.By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of COLIROFTA ANESTHETIC
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging and carton after EXP. The expiration date is the last day of the month indicated.
Before the first opening, store in the refrigerator (between 2°C and 8°C). Once opened, do not store above 25°C.
To avoid infections, discard the bottle 4 weeks after first opening.
Write the opening date of the bottle in the space provided on the carton.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package contents and additional information
Composition of COLIROFTA ANESTHETIC
- The active ingredients are tetracaine hydrochloride and naphazoline hydrochloride. Each ml of eye drops solution contains 5 mg of tetracaine hydrochloride (0.5%) and 0.5 mg of naphazoline hydrochloride (0.05%).
- The other ingredients are chlorobutanol, monosodium phosphate monohydrate, disodium phosphate dodecahydrate, sodium chloride, and purified water.
Appearance of the product and package contents
Colirofta Anesthetic is an eye drop solution; clear and colorless liquid.
It is presented in a carton containing a dropper bottle (10 ml plastic bottle).
Marketing authorization holder and manufacturer
Marketing authorization holder
Alcon Healthcare, S.A.
World Trade Center Almeda Park
Plaça de la Pau s/n, Edificio 6, planta 3
08940 – Cornellà de Llobregat (Barcelona)
Spain
Manufacturer
Siegfried El Masnou, S.A.
C/ Camil Fabra, 58
08320 – El Masnou (Barcelona)
Spain
or
Alcon Laboratories Belgium
Lichterveld 3
2870 Puurs-Sint-Amands
Belgium
Date of the last revision of this leaflet: June 2025
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price3.39 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIROFTA ANESTHETIC 5 mg/ml + 0.5 mg/ml EYE DROPS SOLUTIONDosage form: EYE DROP, 4 mg/ml + 1 mg/mlActive substance: combinationsManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: OPHTHALMIC GEL, 20 mg/gActive substance: lidocaineManufacturer: Laboratoires Doliage DeveloppementPrescription requiredDosage form: EYEDROP, 4 mg/mlActive substance: oxybuprocaineManufacturer: Agepha Pharma S.R.O.Prescription required
Online doctors for COLIROFTA ANESTHETIC 5 mg/ml + 0.5 mg/ml EYE DROPS SOLUTION
Discuss questions about COLIROFTA ANESTHETIC 5 mg/ml + 0.5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















