CLOTIC 10 mg/ml OTIC SOLUTION, SINGLE-DOSE CONTAINERS

How to use CLOTIC 10 mg/ml OTIC SOLUTION, SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Clotic 10mg/ml Ear Drops Solution in Single-Dose Container
clotrimazole
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Clotic and what is it used for
- What you need to know before using Clotic
- How to use Clotic
- Possible side effects
- Storage of Clotic
- Contents of the container and additional information
1. What is Clotic and what is it used for
Clotic is used to treat fungal infections of the outer ear (otomycosis) in adults, adolescents, and children over 1 month.
The active ingredient in this medication is clotrimazole. Clotrimazole belongs to a group of medications called imidazoles and is an antifungal medication that combats the most common types of fungi that cause ear infections.
2. What you need to know before using Clotic
Do not use Clotic
- if you are allergic to clotrimazole or any of the other components of this medication (listed in section 6).
Warnings and precautions
- Use this medication only in the ears. Avoid getting the medication in contact with the eyes. In case of accidental exposure of the eyes to clotrimazole, rinse with plenty of water and consult an ophthalmologist if necessary.
- Consult your doctor before using this medication if you have a perforated eardrum.
Other medications and Clotic
Tell your doctor or pharmacist if you are taking/using, have recently taken/used, or may need to take/use any other medication, including medications dispensed without a prescription.
Pregnancy, breastfeeding, and fertility
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Clotic can be used during breastfeeding.
Driving and using machines
Clotic does not affect the ability to drive vehicles or use machinery.
3. How to use Clotic
Follow exactly the administration instructions of this medication indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
This medication should only be administered in the ear (otic route).
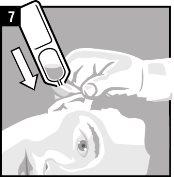
The recommended dose in adults and children is one single-dose container in the affected ear twice a day (morning and night), preferably every 12 hours, for 14 days.
Use this medication in both ears only if your doctor has indicated that you should do so. In that case, use one single-dose container for each ear.
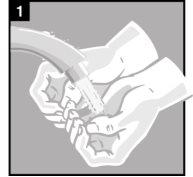
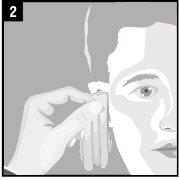
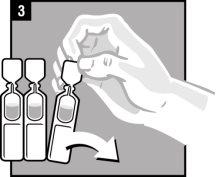
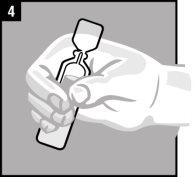
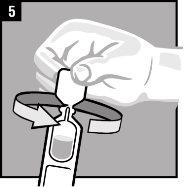
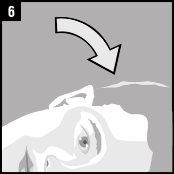
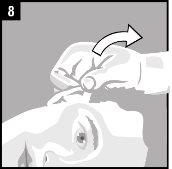
Instructions for use
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Discard the single-dose container after administration. |
|
|
It is essential to follow these instructions for this medication to work.
If you use more Clotic than you should
The symptoms of an overdose are unknown. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Clotic
Do not take a double dose to make up for forgotten doses. If you forget a dose, use this medication as soon as possible and then continue with the rest of your treatment as usual.
If you stop using Clotic
If you stop using this medication before your doctor prescribed, your condition may worsen. To ensure that the infection does not recur, do not stop treatment prematurely, even if your ears feel better. If you need to stop using this medication, consult your doctor immediately.
If you have any further questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone may experience them.
Stop using this medication and inform your doctor immediately if you experience symptoms of an allergic reaction, such as:
- itching, skin rash, or hives,
- swelling of the face, lips, or throat,
- difficulty breathing or wheezing.
Uncommon:may affect up to 1 in 100 people
- Perforated eardrum.
- Ear pain.
- Ringing in the ears (tinnitus).
- Headache.
- Dizziness.
- Sensations such as numbness, tingling, or muscle spasms (paresthesia).
- Pain at the application site.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Agency's website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Clotic
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton after EXP. The expiration date is the last day of the month indicated.
This medication does not require any special storage temperature.
Keep the single-dose containers in the original packaging to protect them from excessive moisture.
Once the bag is opened, the single-dose containers should be used within 30 days and stored below 25°C.
After opening for the first time a single-dose container: use immediately and discard the single-dose container after use.
Medications should not be disposed of via wastewater or household waste. Deposit the containers and medications you no longer need at the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Contents of the container and additional information
Composition of Clotic
- The active ingredient is clotrimazole. Each milliliter of this medication contains 10 mg of clotrimazole. Each single-dose container provides approximately 0.17 ml of solution, equivalent to 1.7 mg of clotrimazole.
- The other component is macrogol.
Appearance of the product and contents of the container
Clotic is a clear and colorless solution supplied in single-dose containers, each containing 0.2 ml of solution.
The single-dose containers are packaged in a protective aluminum bag. Each carton includes 3 bags containing 10 single-dose containers, for a total of 30 single-dose containers in a carton.
Marketing authorization holder and manufacturer
Laboratorios Salvat, S.A.
Gall, 30-36 - 08950
Esplugues de Llobregat (Barcelona)
Spain
Manufacturer
Pharmaloop S.L.
C/Bolivia, 15 – Polig Industrial Azque
28806 Alcalá de Henares,
Madrid – Spain
Or
Laboratorios Salvat, S.A.
Gall, 30-36 - 08950
Esplugues de Llobregat (Barcelona)
Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Austria OtoMyk 10 mg/ml Ohrentropfen, Lösung im Einzeldosisbehältnis
Denmark Clotic 10 mg/ml Øredråber, opløsning i enkeltdosisbeholder
Finland Clotic 10 mg/ml Korvatipat, liuos, kerta-annospakkaus
Germany OtoMyk 10 mg/ml Ohrentropfen, Lösung im Einzeldosisbehältnis
Italy Clotic 10 mg/ml gocce auricolari, soluzione in contenitore monodose
Norway Clotic
Poland Dromyc 10 mg/ml krople do uszu, roztwór w pojemniku jednodawkowym
Portugal Clotic 10 mg/ml gotas auriculares, solução em recipiente unidose
Spain Clotic 10 mg/ml gotas óticas en solución en envase unidosis
Sweden Clotic 10 mg/ml Örondroppar, lösning i endosbehållare
Date of the last revision of this package leaflet:August 2024.
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLOTIC 10 mg/ml OTIC SOLUTION, SINGLE-DOSE CONTAINERSDosage form: OTIC SOLUTION, 3 mg/mlActive substance: ciprofloxacinManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 1.2 mg of ciprofloxacin in 0.4 mlActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 3 mgActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for CLOTIC 10 mg/ml OTIC SOLUTION, SINGLE-DOSE CONTAINERS
Discuss questions about CLOTIC 10 mg/ml OTIC SOLUTION, SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions