
CLEBORIL PEDIATRIC 62.5 micrograms oral drops in solution

How to use CLEBORIL PEDIATRIC 62.5 micrograms oral drops in solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cleboril Pediatric 62.5 micrograms/ml Oral Solution in Drops
clebopride
Read this package leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, consult your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What Cleboril Pediatric is and what it is used for
- What you need to know before taking Cleboril Pediatric
- How to take Cleboril Pediatric
- Possible side effects
- Storage of Cleboril Pediatric
- Contents of the pack and further information
1. What Cleboril Pediatric is and what it is used for
Cleboril Pediatric contains clebopride and belongs to a group of medicines that act on an area of the brain that prevents nausea or vomiting.
Cleboril Pediatric is indicated in children from 1 year of age for the symptomatic treatment of nausea and vomiting, including those induced by antineoplastic chemotherapy.
2. What you need to know before taking Cleboril Pediatric
Do not take Cleboril Pediatric:
- If you are allergic to clebopride or any of the other ingredients of this medicine (listed in section 6).
- If you have bleeding, obstruction, or perforation of the gastrointestinal tract, as stimulation of gastric motility may be harmful.
- If you suffer from abnormal and involuntary movements (tardive dyskinesia) that appear in people being treated with a type of medicine called neuroleptics.
- If you suffer from seizures (epilepsy).
- If you suffer from Parkinson's disease or other extrapyramidal disorders (which cause alterations in muscle tone, posture, and the appearance of involuntary movements).
Warnings and precautions
Consult your doctor or pharmacist before taking Cleboril Pediatric:
- If you have severe liver or kidney disease (severe hepatic or renal insufficiency), as it may increase the effect of this medicine.
- If you have certain tumors, such as breast tumors or prolactin-secreting pituitary adenoma, as it may increase the level of a hormone called prolactin in the blood.
Children and adolescents
In children and adolescents, the use of higher doses than recommended may increase the possibility of extrapyramidal reactions (alterations in muscle tone, posture, and the appearance of involuntary movements).
In newborns, cases of acquired methemoglobinemia (a blood disorder that affects the ability to transport oxygen) due to orthopramides (a group of medicines to which Cleboril Pediatric belongs) have been described.
Other medicines and Cleboril Pediatric
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is especially important that you inform your doctor if you are taking any of the following medicines:
- Phenothiazines, butyrophenones, and other antidopaminergics (used to treat certain mental illnesses) as Cleboril Pediatric may increase the effect of these medicines on the central nervous system.
- Digoxin (used to treat heart diseases) and cimetidine (used in situations where it is necessary to decrease acid production by the stomach) as Cleboril Pediatric decreases their effects.
- Hypnotics (medicines that improve sleep initiation and duration), anxiolytics (reduce anxiety), or narcotics (used to treat moderate or severe pain) as Cleboril Pediatric may potentiate their sedative effects.
- Anticholinergics (such as atropine, used to relieve stomach cramps or spasms, to treat Parkinson's disease, or to prevent travel sickness) or narcotic analgesics (opioids) as they neutralize the action of Cleboril Pediatric on gastrointestinal motility.
- MAOIs (used to treat depression), as their use with Cleboril Pediatric may increase the risk of adverse effects.
Taking Cleboril Pediatric with alcohol
Alcohol should be avoided while being treated with Cleboril Pediatric, as it may potentiate its sedative effects.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is not enough information on the use of this medicine in pregnant or breastfeeding women. As a precaution, it is recommended to avoid its use during pregnancy, especially in the first three months, and breastfeeding.
Driving and using machines
During treatment with Cleboril Pediatric, you should avoid situations that require a special state of alertness, such as driving vehicles or handling dangerous machinery.
Cleboril Pediatric contains benzoic acid (E-210) and sodium.
This medicine contains 1 mg of benzoic acid (E-210) per ml.
This medicine contains less than 1 mmol of sodium (23 mg) per 5 ml dose; i.e., it is essentially "sodium-free".
3. How to take Cleboril Pediatric
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Posology
Use in children only
The recommended dose is 4 to 5 drops of Cleboril Pediatric (12.5 micrograms to 15.62 micrograms) per kg of body weight and day, divided into 3 doses per day.
Generally, the recommended dose is:
From 1 to 4 years: 0.5 ml to 1 ml (10 to 20 drops) 3 times a day.
From 4 to 6 years: 1.5 ml (30 drops) 3 times a day.
From 6 to 8 years: 2 ml (40 drops) 3 times a day.
From 8 to 10 years: 2.5 ml (50 drops) 3 times a day.
From 10 to 12 years: 3 ml (60 drops) 3 times a day.
Method of administration
This medicine is for oral administration.
The drops will be administered directly or dissolved in half a glass of water, before the main meals.
Use only the dosing device provided with the package. Do not use any type of spoon.
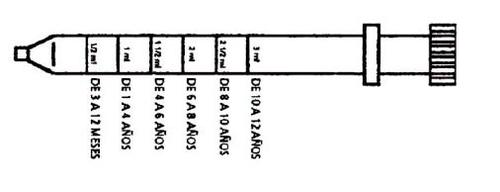
Dosing device for children and infants
If you take more Cleboril Pediatric than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone 91 5620420, indicating the medicine and the amount taken.
In case of overdose, drowsiness, disorientation, and extrapyramidal reactions (alterations in muscle tone, posture, and the appearance of involuntary movements) may appear, which normally disappear when treatment is discontinued.
If symptoms persist, gastric lavage and symptomatic medication will be performed. Extrapyramidal reactions are controlled with the administration of medicines for Parkinson's disease.
If you forget to take Cleboril Pediatric
Do not take a double dose to make up for forgotten doses.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported in clinical trials and during post-marketing experience:
Rare side effects (may affect up to 1 in 1,000 patients)
- Alterations in muscle tone, posture, and the appearance of involuntary movements (extrapyramidal disorders)
- Parkinsonism
- Abnormal and involuntary movements (dystonias, most frequently reported in the neck, tongue, or face; dyskinesia; tardive dyskinesia, in elderly patients after prolonged treatments).
- Sedation
- Tremor
- Drowsiness
Very rare side effects (may affect up to 1 in 10,000 patients)
- Increased levels of prolactin in the blood (hyperprolactinemia)
- Milk secretion outside of the lactation period (galactorrhea)
- Increased breast size in males (gynecomastia)
- Difficulty achieving or maintaining an erection (erectile dysfunction)
- Absence of menstruation (amenorrhea)
These side effects have been reported after prolonged treatments.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cleboril Pediatric
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiry date which is stated on the package after EXP. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Cleboril Pediatric
- The active substance is clebopride. Each ml (20 drops) contains 62.5 micrograms of clebopride (as clebopride malate).
- The other ingredients (excipients) are benzoic acid (E-210), sodium hydroxide, and purified water.
Appearance of the product and pack contents
Cleboril Pediatric is presented as a colorless, transparent, and homogeneous solution with a characteristic odor, in amber glass bottles with a "Pilfer Proof" plastic cap, 90 ml capacity, and a dosing syringe.
Marketing authorization holder and manufacturer
Marketing authorization holder
Almirall, S.A.
General Mitre, 151
08022 – Barcelona
Manufacturer
Industrias Farmacéuticas Almirall, S.A.
Ctra. de Martorell, 41-61
08740 Sant Andreu de la Barca - Barcelona (Spain)
or
Manufacturer
Almirall Hermal GmbH
Scholtzstraße, 3 21465
Reinbek Germany
Date of the last revision of this package leaflet:November 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price1.53 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLEBORIL PEDIATRIC 62.5 micrograms oral drops in solutionDosage form: ORAL SOLUTION/SUSPENSION, 10 mg clebopride/100 mlActive substance: cleboprideManufacturer: Almirall S.A.Prescription requiredDosage form: TABLET, 500 mcg CleboprideActive substance: cleboprideManufacturer: Almirall S.A.Prescription requiredDosage form: CAPSULE, 0.5 mg clebopride + 200 mg simethiconeActive substance: cleboprideManufacturer: Almirall S.A.Prescription required
Online doctors for CLEBORIL PEDIATRIC 62.5 micrograms oral drops in solution
Discuss questions about CLEBORIL PEDIATRIC 62.5 micrograms oral drops in solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















