
CICLONIC 80 MG/G NAIL LACQUER MEDICATED

How to use CICLONIC 80 MG/G NAIL LACQUER MEDICATED
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ciclonic 80 mg/g Medicinal Nail Varnish
Ciclopirox
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Ciclonic and what is it used for
- What you need to know before you start using Ciclonic
- How to use Ciclonic
- Possible side effects
- Storage of Ciclonic
- Contents of the pack and further information
1. What is Ciclonic and what is it used for
Ciclonic 80 mg/g is a medicinal nail varnish. It contains the active substance ciclopirox, which belongs to a group of medicines known as antifungals. Ciclopirox penetrates the nail plate and has a fungicidal effect on all major causative agents of nail fungus infections.
Ciclonic 80 mg/g is used to treat fungal infections of the nails (onychomycosis).
2. What you need to know before you start using Ciclonic
Do not use Ciclonic:
- if you are allergic to ciclopirox or any of the other ingredients of this medicine (listed in section 6).
- in children due to insufficient experience in this age group to date.
- during pregnancy and breastfeeding
Warnings and precautions
Talk to your doctor or pharmacist before you start using Ciclonic.
The use, especially if prolonged, of topical products may lead to sensitization phenomena or cause adverse reactions. In these situations, treatment should be discontinued and appropriate therapeutic measures taken. Contact with the eyes and mucous membranes should be avoided.
Do not use nail varnish or other nail cosmetic products during treatment with Ciclonic.
Children and adolescents
Due to inadequate clinical experience, Ciclonic 80 mg/g medicinal nail varnish should not be used in children.
Other medicines and Ciclonic
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines.
Using Ciclonic with food, drinks, and alcohol
Not applicable.
Pregnancy, breastfeeding, and fertility
Ciclonic is generally contraindicated during pregnancy and breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines
Ciclonic has no or negligible influence on the ability to drive and use machines.
3. How to use Ciclonic 80 mg/g medicinal nail varnish
Follow the instructions for administration of this medicine exactly as described in this package leaflet or as directed by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Unless otherwise instructed, apply a thin layer of Ciclonic medicinal nail varnish to the affected nail once a day.
Presentation without nail files included
Before the first application of Ciclonic, remove as much as possible of the affected part of the nail, for example, with nail scissors, and as much as possible of the hyperkeratotic material with a commercially available disposable nail file.
Presentation with nail files included
Before the first application of Ciclonic, remove as much as possible of the affected part of the nail, for example, with nail scissors, and as much as possible of the hyperkeratotic material with one of the included disposable nail files.
During the entire application period, once a week, remove the entire layer of varnish with a commercially available cotton swab dipped in alcohol. Also, during this process, remove as much as possible of the hyperkeratotic material from the nail plate, always with a disposable nail file.
If the varnish layer is damaged between applications, simply reapply Ciclonic to the affected areas.
Treatment duration depends on the severity of the infection but should not exceed 6 months.
After each use, it is recommended to close the bottle cap tightly to prevent the solution from drying out.
Do not let the solution come into contact with the neck of the bottle to avoid the cap sticking.
If you use more Ciclonic than you should:
No cases of overdose have been reported.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Ciclonic:
Do not use a double dose to make up for forgotten doses. Continue treatment as recommended by your doctor or as explained in section 3 of this package leaflet (How to use Ciclonic).
If you stop using Ciclonic:
If you stop using Ciclonic before your nails are completely cleared or their appearance has improved substantially and healthy nails have regrown, it is possible that the fungus has not been completely eliminated. In such cases, the onychomycosis may worsen again.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency of possible side effects listed below is defined as:
Very common: affects more than 1 in 10 people.
Common: affects between 1 and 10 in 100 people.
Uncommon: affects between 1 and 10 in 1,000 people.
Rare: affects between 1 and 10 in 10,000 people.
Very rare: affects less than 1 in 10,000 people.
Frequency not known: cannot be estimated from the available data.
Rarely, allergic dermatitis has been observed when the skin surrounding the nail has come into contact with Ciclonic. In very rare cases, redness and desquamation have been observed.
Following the instructions in this package leaflet reduces the risk of side effects.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ciclonic
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
After first opening, use within 6 months. Keep the bottle tightly closed to prevent the contents from evaporating.
Do not use this medicine after the expiry date stated on the carton after ‘EXP’. The expiry date is the last day of the month stated.
This product is flammable. Keep away from heat and open flames.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy’s SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Ciclonic
The active substance is ciclopirox. 1 gram of medicinal nail varnish contains 80 mg of ciclopirox.
The other ingredients are methoxyethylene polymer with 2-butenedioic acid, monobutyl ester, 1:1, ethyl acetate, and isopropyl alcohol.
Appearance and packaging of the product:
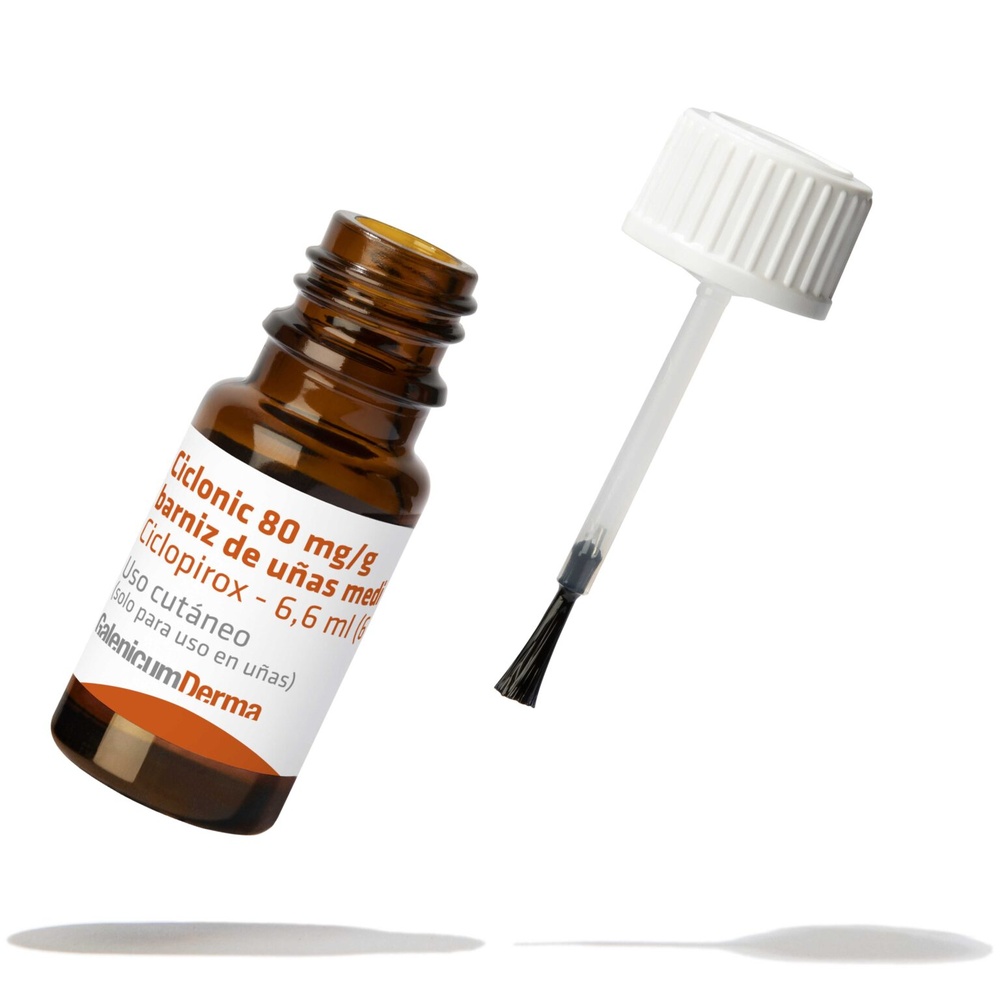
Ciclonic is a clear, colorless, or slightly yellowish solution presented in an amber glass bottle with a screw cap equipped with a brush.
The pack sizes are 3.3 ml (3g), 6.6ml (6g), 3.3 ml (3g) with 30 disposable nail files, and 6.6 ml (6g) with 30 disposable nail files.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Galenicum Derma, S.L.U.
Ctra. N-1, Km 36
San Agustin del Guadalix
+34 932 011 750
Manufacturers
Doppel Farmaceutici S.R.L.
Via Martiri delle Foibe, 1
29016 Cortemaggiore (PC)
Italy
Qualimetrix S.A.
579 Mesogeion Avenue
Agia Paraskevi
Athens 15343
Greece
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Poland: Axopirox 8% w/w lakier do paznokci, leczniczy
Slovakia: Ciclopirox AXXON 80mg/g liecivý lak na nechty
Spain: Ciclonic 80mg/g barniz de uñas medicamentoso
Hungary: Kitolak 8 % gyógyszeres körömlakk
Estonia: Ciclopirox Terix
Date of the last revision of this package leaflet: August 2024
- Country of registration
- Average pharmacy price16.59 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CICLONIC 80 MG/G NAIL LACQUER MEDICATEDDosage form: CREAM, 10 mgActive substance: ciclopiroxManufacturer: Galenicum Derma S.L.U.Prescription requiredDosage form: SHAMPOO, 1.5% ciclopirox olamineActive substance: ciclopiroxManufacturer: Galenicum Derma S.L.U.Prescription requiredDosage form: NAIL POLISH, 8% ciclopiroxActive substance: ciclopiroxManufacturer: Galenicum Derma S.L.U.Prescription required
Online doctors for CICLONIC 80 MG/G NAIL LACQUER MEDICATED
Discuss questions about CICLONIC 80 MG/G NAIL LACQUER MEDICATED, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








