
CAVERJECT 10 micrograms POWDER AND SOLVENT FOR INJECTION

How to use CAVERJECT 10 micrograms POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Caverject 10 micrograms powder and solvent for solution for injection
Caverject 20 micrograms powder and solvent for solution for injection
Alprostadil
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Caverject and what is it used for
- What you need to know before you use Caverject
- How to use Caverject
- Possible side effects
5 Storage of Caverject
- Contents of the pack and further information
1. What is Caverject and what is it used for
Caverject is a prostaglandin (E1) with vasodilatory action.
Caverject is indicated for the treatment of erectile dysfunction in adult males, including insufficient erections or impotence. Your doctor may also use it, along with other tests, to find the exact cause of your erectile dysfunction.
2. What you need to know before you use Caverject
Do not use Caverject
- if you are allergic to alprostadil or any of the other ingredients of this medicine (listed in section 6).
- if you have sickle cell anemia (a disease that affects red blood cells), multiple myeloma (a form of bone marrow cancer, in which there is an abnormal proliferation of plasma cells, which are the blood cells that produce antibodies, which defend us against infections and other foreign substances), leukemia (a disease of the bone marrow that causes an uncontrolled increase in white blood cells), drepanocytic trait or drepanocytic anemia (a disease that is transmitted from parents to children and in which red blood cells have an abnormal semilunar shape), thrombocytopenia (a disease characterized by a decrease in the number of platelets in the blood below normal levels), polycythemia (a disease characterized by an increase in hematocrit, the proportion of red blood cells in the blood), if you have a predisposition to suffer from venous thrombosis or any other disease that favors the appearance of priapism (very prolonged erection).
- if you have an anatomical deformation of the penis, such as angulation, cavernous fibrosis, or Peyronie's disease.
- if you have a penile implant.
- if you suffer from a disease (such as very severe heart disease) in which sexual activity is discouraged or contraindicated.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Caverject:
- if you or your partner have a sexually transmitted disease, such as AIDS, herpes, or gonorrhea. The injection of Caverject can produce small bleeding at the injection site, which could increase the risk of transmitting these diseases. The use of Caverject does not protect you from sexually transmitted diseases, so you must inform yourself about the necessary measures to avoid contagion of this type of sexually transmitted disease.
- if you are being treated with anticoagulants such as warfarin or heparin, as bleeding may occur after injection.
- if priapism (prolonged erection) appears during treatment, which should be reported to your doctor.
- if you have had a stroke or suffer from any heart, blood vessel, or lung disorder.
- if you have one or more cardiovascular risk factors (these may include
arterial hypertension, smoking, elevated blood glucose, elevated blood cholesterol, overweight, and obesity).
- if you have one or more risk factors for stroke (these may include
arterial hypertension, elevated blood cholesterol, coronary artery disease, irregular heart rhythm, diabetes).
- if the formation of a hardening (plaque) occurs in an area of the penis that produces the curvature of the penis towards the side where the hard area is located (fibrosis, Peyronie's disease), which should be immediately reported to your doctor.
- if you have a history of psychiatric disorders or addiction.
Children and adolescents
This is not applicable.
Using Caverject with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Do not use other medicines for erectile dysfunction at the same time as Caverject.
Certain medicines may interact with Caverject; in these cases, it may be necessary to change the dose or interrupt treatment with one of them.
It is important that you inform your doctor if you are taking or have recently taken any of the following medicines:
- anticoagulants, such as warfarin or heparin, as you may bleed more when performing the injection.
- antihypertensives, vasodilators, anticoagulants, and platelet aggregation inhibitors, as Caverject could increase the effects of these medicines.
- sympathomimetics, as these medicines could reduce the effect of Caverject.
Taking Caverject with food, drinks, and alcohol
This is not applicable.
Pregnancy, breastfeeding, and fertility
Caverject is not indicated in women.
Driving and using machines
It is not expected that the ability to drive or operate machinery will be affected as a result of the administration of Alprostadil. Some patients treated with Caverject have suffered some episodes of low blood pressure and fainting (syncope), so while you are being treated with this medicine, it is recommended that you avoid situations in which you may be injured, including driving or using hazardous machinery.
Caverject contains benzyl alcohol and sodium
Once reconstituted, this medicine contains 8.4 mg of benzyl alcohol per ml. Benzyl alcohol may cause allergic reactions. Consult your doctor or pharmacist if you have liver or kidney disease. This is because large amounts of benzyl alcohol can accumulate in the body and cause adverse effects (metabolic acidosis).
This medicine contains less than 23 mg of sodium (1 mmol) per ml, i.e., it is essentially "sodium-free".
3. How to use Caverject
Follow the instructions for administration of this medicine exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Your doctor or nurse will teach you how to perform the injection. Do not attempt to do it on your own until you have learned the correct way to administer it. If you do not remember how to do it, consult your doctor again. These instructions are only a reminder.
Each patient needs a different dose of Caverject for the treatment of erectile dysfunction.
Perform an injection of the dose prescribed by your doctor (5 to 20 micrograms of alprostadil). If you need an additional dose adjustment, you should consult your doctor. The maximum daily dose is 60 micrograms of alprostadil.
The frequency of administration is no more than once a day and no more than three times a week. Do not use Caverject more often. Each time you use Caverject, change the side of the penis and the exact site where you perform the injection.
Caverject (sterile lyophilized) is packaged in 5 ml capacity vials. For its reconstitution, the solvent contained in the attached syringe must be used.
After reconstitution by adding 1 ml of solvent, the resulting solution contains 10 micrograms or 20 micrograms of alprostadil per ml. (See Storage of Caverject).
The reconstituted alprostadil solutions are for single use. The syringe and remaining solution should be discarded properly.
Erection usually occurs between 5 and 15 minutes after injection. The duration of the erection depends on the administered dose. The selected dose for treatment should provide you with an erection that allows you to have satisfactory sexual intercourse, maintained for no more than 60 minutes. If the duration of the erection is more than 60 minutes, a dose reduction will be necessary. Consult your doctor in case of prolonged erection.
The patient will attend the doctor's office every three months for follow-up of the self-administration therapy. The reconstituted vial, syringe, and needles are intended for single use and should be discarded after use.
Instructions for correct administration:
Dilution and extraction of the medication
- Wash your hands with water and soap.
- Remove the plastic cap from the vial.
- Clean the rubber stopper of the vial using one of the alcohol-impregnated wipes provided (the other will be needed later).
- Remove the protective film from the larger needle (labeled with 22G1½) while keeping the plastic sheath in place. Turn the top of the tamper-evident white cap by breaking the seal. Place the needle on the syringe, turning it towards the neck of the syringe.
- Puncture the needle through the central portion of the rubber stopper of the vial and introduce all of the solvent into it.
- Handle the syringe and vial carefully as a unit, shake until the powder is completely dissolved.
- To extract the medication, place the vial with the syringe punctured in an inverted position. Slowly pull the plunger of the syringe until the solution reaches the level recommended by your doctor.
- Tap the syringe to eliminate any possible bubbles or inject the solution back into the vial and extract it again slowly.
- Remove the needle and syringe from the vial.
- Replace the needle with the smaller one (labeled with 30G½) to perform the self-injection.
Self-injection
- Undress and get comfortable. If your doctor has recommended that you use alcohol cleaning wipes, open one.
- Make sure the needle is not bent. If it is, do not use it and throw it away. Do not try to straighten it.
- The solution will be injected into either of the two areas of the penis called the corpora cavernosa. As shown in diagram A, the corpora cavernosa are located along the shaft (diaphysis) of the penis, one on each side.
Each time you use Caverject, alternate the injection sites: choose one side for this injection and the other for the next time. Within each area, the injection site should also be changed each time.
Hold the penis, placing the index and middle fingers underneath, near the testicles, and the thumb on top. Gently squeeze the penis between the thumb and fingers so that the injection site protrudes. If you have a foreskin, make sure it is stretched.
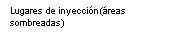
- Using the alcohol-impregnated wipe, clean the skin of that area and let it dry.
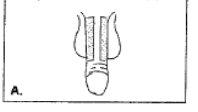
- Holding the penis firmly with one hand and holding the syringe with the other, puncture the swollen area with the needle, passing through the skin with a continuous movement. Avoid visible veins and blood vessels. Diagrams B and C show the correct angle, which should be 90 degrees. Push the plunger firmly. If the solution does not pass easily, move the needle slightly and try again. Do not force the passage of the Caverject liquid through the needle.


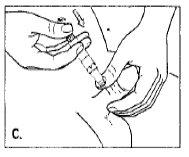
- Remove the needle. Gently press the alcohol-impregnated wipe on the puncture site for 3 minutes. If bleeding appears, maintain pressure until it disappears. Massage the penis to help distribute the alprostadil.
- Do not save what is left in the cartridge for a second injection. When you have finished with the needle, carefully throw it away, as your doctor has recommended, so that no one sees it, uses it, or pricks themselves with it.
If you think the action of Caverject is too strong or too weak, tell your doctor or pharmacist.
If you use more Caverject than you should
If you have used more Caverject than you should and have an erection that lasts more than four hours, rapid breathing, feel weak or dizzy, depressed, with loose stools or diarrhea, consult your doctor or pharmacist immediately, as you may need treatment.
In case of overdose or accidental administration, consult the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount administered.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported with the approximate frequencies indicated:
Very common (may affect more than 1 in 10 people)
Common (may affect up to 1 in 10 people)
Uncommon (may affect up to 1 in 100 people)
Rare (may affect up to 1 in 1,000 people)
Frequency not known (cannot be estimated from the available data)
Very common (may affect more than 1 in 10 people):
- pain in the penis.
Common (may affect up to 1 in 10 people):
- bruise (hematoma).
- redness of the skin (erythema).
- cramps.
- scarring and angulation of the penis (fibrosis, angulation, fibrotic nodules, and Peyronie's disease), penile disorder, prolonged erection, in most cases, it was reduced spontaneously.
- redness at the injection site due to blood extravasation into the tissues (ecchymosis), hematoma at the injection site, which have been related to the injection technique rather than the effect of Caverject.
Uncommon (may affect up to 1 in 100 people):
- fungal infection, common cold symptoms.
- feeling of diminished consciousness, without losing it (presyncope), decreased touch sensation (hypoesthesia), exaggerated sensation of tactile stimuli (hyperesthesia).
- increased sensitivity of the eyes to light (mydriasis).
- a heart rhythm disorder known as supraventricular extrasystole, increased heart rate.
- hypotension (including symptomatic hypotension), increased blood vessel caliber (vasodilation), peripheral vascular disorders, venous disorder.
- nausea, dry mouth.
- rash, increased sweating, itching.
- if Caverject is accidentally injected into the urethra (the duct through which urine leaves the penis), blood may appear in the urine or at the tip of the penis.
- need to urinate more frequently than normal, even with urgency and pain, difficulty urinating.
- prolonged erection for more than six hours (priapism), pain in the pelvis, swelling that can be felt in the scrotum (spermatocele), testicular disorders (pain, fluid accumulation, swelling), scrotal disorders (pain, fluid accumulation), redness of the scrotum, painful erection, inflammation of the glans (balanitis), phimosis, repeated inability to achieve or maintain an erection sufficient for satisfactory sexual intercourse (erectile dysfunction), abnormal ejaculation.
- bleeding, hemorrhage at the injection site, inflammation, inflammation at the injection site, feeling of heat at the injection site, fluid accumulation (edema) at the injection site, swelling at the injection site, pain at the injection site, irritation at the injection site, generalized weakness (asthenia), numbness of the injection site (anesthesia), fluid accumulation (edema), venous fluid accumulation, itching at the injection site.
- increased creatinine in the blood.
Frequency not known (cannot be estimated from the available data):
- Headache
- Increased blood pressure (hypertension)
- Back pain
- Localized pain (buttocks, legs, genitals), buttock weakness.
- Insufficient blood flow to the heart muscle through the coronary arteries
- Stroke
If the erection lasts more than 4 hours, consult your doctor immediately. If you cannot contact your doctor, go to the emergency department of a hospital.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Caverject
Keep this medicine out of the sight and reach of children.
This medicine does not require special storage conditions.
Do not use Caverject after the expiration date that appears on the packaging after CAD. The expiration date is the last day of the month indicated.
The reconstituted solution should be used immediately. Do not use the resulting solution if it appears cloudy, discolored, or contains particles.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medicines at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Caverject
- The active ingredient is alprostadil.
- The other components are:
In the lyophilisate: lactose monohydrate, alpha cyclodextrin (Alfadex), sodium citrate dihydrate, sodium hydroxide (pH adjustment), hydrochloric acid (pH adjustment).
In the solvent: water for injectable preparations in solution with benzyl alcohol (E1519) at 0.9%
Appearance of the Product and Package Contents
Caverject 10 micrograms powder and solvent for injectable solution is presented in a single format of 1 vial with a capacity of 5 ml containing 10 micrograms of sterile lyophilized powder and a pre-filled syringe with a capacity of 2.5 ml containing 1 ml of solvent.
Caverject 20 micrograms powder and solvent for injectable solution is presented in a single format of 1 vial with a capacity of 5 ml containing 20 micrograms of sterile lyophilized powder and a pre-filled syringe with a capacity of 2.5 ml containing 1 ml of solvent.
In both cases, the packaging also contains the following materials:
- 30G needle with a length of 1/2 inch (used for injecting the drug).
- 22G needle with a length of 1 1/2 inches (used for reconstituting the drug)
- 2 alcohol-impregnated swabs.
Marketing Authorization Holder and Manufacturer
- Holder:
Pfizer, S.L.
Avda. de Europa 20-B
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid)
SPAIN
- Manufacturer:
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
Date of the Last Revision of this Leaflet: June 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CAVERJECT 10 micrograms POWDER AND SOLVENT FOR INJECTIONDosage form: CREAM, 300 micrograms of alprostadil in 100 mg of cream (0.3% w/w)Active substance: alprostadilManufacturer: Recordati Ireland LimitedPrescription requiredDosage form: INJECTABLE, 20 mcg alprostadilActive substance: alprostadilManufacturer: Pfizer S.L.Prescription requiredDosage form: CREAM, 300 MCG (0.2% W/W) Alprostadil 100 mg CreamActive substance: alprostadilManufacturer: Recordati Ireland LimitedPrescription required
Online doctors for CAVERJECT 10 micrograms POWDER AND SOLVENT FOR INJECTION
Discuss questions about CAVERJECT 10 micrograms POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






