
BRIVIACT 10 mg/ml ORAL SOLUTION

How to use BRIVIACT 10 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
Briviact 10 mg/ml Oral Solution
brivaracetam
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Briviact and what is it used for
- What you need to know before you take Briviact
- How to take Briviact
- Possible side effects
- How to store Briviact
- Contents of the pack and other information
1. What is Briviact and what is it used for
What is Briviact
Briviact contains the active substance brivaracetam. It belongs to a group of medicines called “antiepileptics”. These medicines are used to treat epilepsy.
What Briviact is used for
- Briviact is used in adults, adolescents and children from 2 years of age.
- It is used to treat a type of epilepsy that causes partial seizures with or without secondary generalisation.
- Partial seizures are seizures that affect only one side of the brain. These partial seizures can spread to larger areas on both sides of the brain – this is called “secondary generalisation”.
- Your doctor has prescribed this medicine to reduce the number of seizures you have.
- Briviact is used together with other medicines to treat epilepsy.
2. What you need to know before you take Briviact
Do not take Briviact
- if you are allergic to brivaracetam, to other similar chemical compounds such as levetiracetam or piracetam or to any of the other ingredients of this medicine (listed in section 6). If you are not sure, consult your doctor or pharmacist before taking Briviact.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Briviact:
- If you have had thoughts of harming yourself or suicide. A small number of people taking antiepileptic medicines such as Briviact have had thoughts of harming themselves or suicide. If you have any of these thoughts, contact your doctor immediately.
- If you have liver problems, your doctor may need to adjust your dose.
Children
Briviact is not recommended for use in children below 2 years of age.
Taking Briviact with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, tell your doctor if you are taking any of the following medicines, because your doctor may need to adjust your dose of Briviact:
- Rifampicin, a medicine used to treat bacterial infections.
- St John’s Wort (also known as Hypericum perforatum), a herbal medicine used to treat depression and anxiety, as well as other conditions.
Taking Briviact with alcohol
- It is not recommended to take this medicine with alcohol.
- If you drink alcohol while taking Briviact, the negative effects of alcohol may increase.
Pregnancy and breast-feeding
Women of childbearing potential should discuss the use of contraceptives with their doctor.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
It is not recommended to take Briviact if you are pregnant, as the effects of Briviact on pregnancy and the unborn child are not known.
It is not recommended to breast-feed while taking Briviact, as Briviact is excreted in breast milk.
Do not stop treatment without first consulting your doctor. Stopping treatment may increase the number of seizures you have and harm your baby.
Driving and using machines
- You may feel drowsy, dizzy or tired while taking Briviact.
- These effects are more common at the start of treatment or after a dose increase.
- Do not drive, ride a bicycle or use any tools or machinery until you know how this medicine affects you.
Briviact oral solution containsmethyl parahydroxybenzoate, sodium, sorbitol and propylene glycol
- Methyl parahydroxybenzoate (E218): may cause allergic reactions (possibly delayed).
- Sodium: this medicine contains less than 23 mg of sodium (1 mmol) per millilitre; this is, essentially, “sodium-free”.
- Sorbitol (E420) (a type of sugar): this medicine contains 168 mg of sorbitol in each millilitre. Sorbitol is a source of fructose. If your doctor has told you (or your child) that you (or your child) have an intolerance to some sugars, or have been diagnosed with a rare genetic disorder called hereditary fructose intolerance (HFI), in which the patient cannot break down fructose, consult your (or your child’s) doctor before taking this medicine.
- Propylene glycol (E1520): this medicine contains a maximum of 5.5 mg of propylene glycol in each millilitre.
3. How to take Briviact
Follow exactly the instructions given to you by your doctor. If you are not sure, consult your doctor or pharmacist again.
You will take Briviact together with other medicines to treat epilepsy.
Dose
Your doctor will calculate the correct daily dose for you. Take the daily dose in two equal doses, one in the morning and one in the evening, approximately 12 hours apart.
Adolescents and children weighing 50 kg or more, and adults
- The recommended dose is between 25 mg and 100 mg twice a day. Your doctor may later decide to adjust it to find the best dose for you.
The following table only shows examples of doses to be taken and which syringe to use. Your doctor will determine the correct dose for you and which syringe to use, based on your weight.
Dose in ml to be taken twice a day and which syringe to use: for adolescents and children weighing 50 kg or more, and adults: | ||||
Weight | Dose in ml (corresponding to 25 mg = 2.5 ml) | Dose in ml (corresponding to 50 mg = 5 ml) | Dose in ml (corresponding to 75 mg = 7.5 ml) | Dose in ml (corresponding to 100 mg = 10 ml) |
50 kg or more | 2.5 ml | 5 ml | 7.5 ml | 10 ml |
Use the 5 ml syringe (blue graduation marks) | Use the 10 ml syringe (black graduation marks) |
Adolescents and children weighing from 20 kg to less than 50 kg
- The recommended dose is between 0.5 mg and 2 mg per kilogram of body weight twice a day. Your doctor may later decide to adjust it to find the best dose for you.
The following table only shows examples of doses to be taken and which syringe to use. Your doctor will determine the correct dose for you and which syringe to use, based on your weight.
Dose in ml to be taken twice a day and which syringe to use: for adolescents and children weighing from 20 kg to less than 50 kg: | ||||
Weight | Dose in ml (corresponding to 0.5 mg/kg = 0.05 ml/kg) | Dose in ml (corresponding to 1 mg/kg = 0.1 ml/kg) | Dose in ml (corresponding to 1.5 mg/kg = 0.15 ml/kg) | Dose in ml (corresponding to 2 mg/kg = 0.2 ml/kg) |
20 kg | 1 ml | 2 ml | 3 ml | 4 ml |
25 kg | 1.25 ml | 2.5 ml | 3.75 ml | 5 ml |
30 kg | 1.5 ml | 3 ml | 4.5 ml | 6 ml* |
35 kg | 1.75 ml | 3.5 ml | 5.25 ml* | 7 ml* |
40 kg | 2 ml | 4 ml | 6 ml* | 8 ml* |
45 kg | 2.25 ml | 4.5 ml | 6.75 ml* | 9 ml |
Use the 5 ml syringe (blue graduation marks) | For volumes between 0.5 ml and 5 ml, use the 5 ml oral syringe (blue graduation marks)
|
Children weighing from 10 kg to less than 20 kg
- The recommended dose is 0.5 mg to 2.5 mg per kilogram of body weight, twice a day. Your child’s doctor may decide to adjust the dose to find the best dose for your child.
The following table only shows examples of doses to be taken and which syringe to use. Your doctor will determine the correct dose for you and which syringe to use, based on your weight.
Dose in ml to be taken twice a day and which syringe to use for children weighing from 10 kg to less than 20 kg: | |||||
Weight | Dose in ml (corresponding to 0.5 mg/kg = 0.05 ml/kg) | Dose in ml (corresponding to 1.25 mg/kg = 0.125 ml/kg) | Dose in ml (corresponding to 1.5 mg/kg = 0.15 ml/kg) | Dose in ml (corresponding to 2 mg/kg = 0.2 ml/kg) | Dose in ml (corresponding to 2.5 mg/kg = 0.25 ml/kg) |
10 kg | 0.5 ml | 1.25 ml | 1.5 ml | 2 ml | 2.5 ml |
12 kg | 0.6 ml | 1.5 ml | 1.8 ml | 2.4 ml | 3.0 ml |
14 kg | 0.7 ml | 1.75 ml | 2.1 ml | 2.8 ml | 3.5 ml |
15 kg | 0.75 ml | 1.9 ml | 2.25 ml | 3 ml | 3.75 ml |
Use the 5 ml syringe (blue graduation marks) |
Patient with liver problems
If you have liver problems:
- As an adolescent or child weighing 50 kg or more, or as an adult, the maximum dose you will take is 75 mg twice a day.
- As an adolescent or child weighing from 20 kg to less than 50 kg, the maximum dose you will take is 1.5 mg per kilogram of body weight twice a day.
As a child weighing from 10 kg to less than 20 kg, the maximum dose your child will take is 2 mg per kilogram of body weight twice a day.
How to take Briviact oral solution
- You can take Briviact oral solution on its own or diluted in water or juice immediately before swallowing.
- The medicine can be taken with or without food.
Instructions for use by patients or caregivers:
The box includes two oral syringes. Check with your doctor which one to use.
- For a volume between 0.5 ml and 5 ml, you must use the 5 ml oral syringe (blue graduation marks) supplied with the box to ensure accurate dosing.
- For volumes greater than 5 ml and up to 10 ml, you must use the 10 ml oral syringe (black graduation marks) supplied with the box to ensure accurate dosing.
5 ml oral syringe | 10 ml oral syringe |
The 5 ml oral syringe has 2 superimposed blue graduation marks: at intervals of 0.25 ml and 0.1 ml. | The 10 ml oral syringe has black graduation marks at intervals of 0.25 ml. |
- Open the bottle: press the cap and unscrew it in the opposite direction of the clock hands (figure 1).
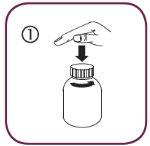
Follow these steps the first time you take Briviact:
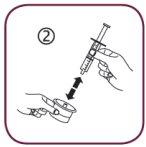
- Separate the syringe adapter (figure 2).
- Insert the adapter into the neck of the bottle (figure 3). Make sure it is well fixed. You do not need to remove the adapter after use.
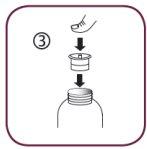
Follow these steps each time you take Briviact:
- Place the oral syringe in the adapter opening (figure 4).
- Put the bottle upside down (figure 5).
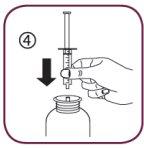
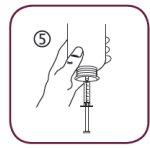
- Hold the bottle upside down with one hand and use the other to fill the oral syringe.
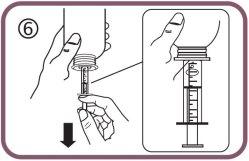
- Lower the plunger to fill the oral syringe with a small amount of solution (figure 6).
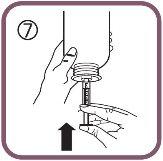
- Then, raise the plunger to eliminate any possible bubbles (figure 7).
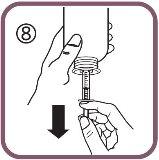
- Lower the plunger to the dose mark in millilitres (ml) on the oral syringe prescribed by your doctor (figure 8).
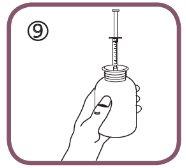
- Put the bottle back upright (figure 9).
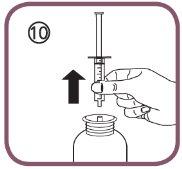
- Remove the oral syringe from the adapter (figure 10).
You can choose between two ways to take the medicine:
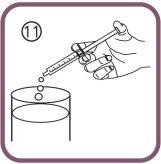
- empty the contents of the syringe into water (or juice) by pushing the plunger to the bottom of the oral syringe (figure 11) – then you will have to drink all the water (add only enough to make it easy to drink) or
- drink the solution directly from the oral syringe without water – drink all the contents of the syringe (figure 12).
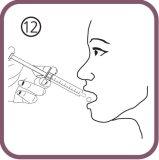
- Close the bottle with the plastic screw cap (you do not need to remove the adapter).
- To clean the oral syringe, rinse it only with cold water, moving the plunger several times up and down to collect and expel the water, without separating the two components of the syringe (figure 13).
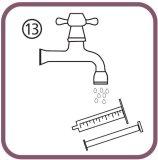
- Store the bottle, the oral syringe and the leaflet in the box.
Duration of treatment with Briviact
Briviact is a long-term treatment – continue taking Briviact until your doctor tells you to stop.
If you take more Briviact than you should
If you take more Briviact than you should, consult your doctor. You may feel dizzy and drowsy.
You may also experience some of the following symptoms: feeling unwell, feeling like you are spinning, problems keeping your balance, anxiety, feeling very tired, irritability, aggression, difficulty sleeping, depression, thoughts or attempts to harm yourself or suicide.
If you forget to take Briviact
- If you forget to take a dose, take it as soon as you remember.
- Take your next dose at the time you would normally take it.
- Do not take a double dose to make up for a forgotten dose.
- If you are not sure what to do, consult your doctor or pharmacist.
If you stop taking Briviact
- Do not stop treatment unless your doctor tells you to. This is because stopping treatment may increase the number of seizures you have.
- If your doctor decides to stop your treatment, he/she will give you instructions for the gradual withdrawal of Briviact. This will help prevent your seizures from coming back or getting worse.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Very Common:may affect more than 1 in 10 patients.
- drowsiness or dizziness.
Common:may affect up to 1 in 10 patients.
- flu
- feeling very tired (fatigue)
- seizure, feeling of spinning (vertigo)
- feeling of nausea and vomiting, constipation
- depression, anxiety, difficulty sleeping (insomnia), irritability
- nose and throat infections (such as "common cold"), cough
- decreased appetite
Uncommon:May affect up to 1 in 100 patients
- allergic reactions
- abnormal thoughts and/or loss of contact with reality (psychotic disorder), aggression, nervousness (agitation)
- thoughts or attempts to harm oneself or commit suicide: inform your doctor immediately
- a decrease in white blood cells (called 'neutropenia') - which appears in blood tests
Other Adverse Effects in Children
Common:may affect up to 1 in 10 patients.
- restlessness and hyperactivity (psychomotor hyperactivity)
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Briviact
- Keep this medicine out of sight and reach of children.
- Do not use this medicine after the expiration date that appears on the packaging and the bottle after CAD. The expiration date is the last day of the month indicated.
- This medicine does not require special storage conditions.
- After the first opening of the bottle, use before 8 months.
- Medicines should not be thrown down the drain or into the trash. Ask your pharmacist how to dispose of the packaging and medicines that you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Briviact
- The active ingredient is brivaracetam.
- Each milliliter (ml) contains 10 milligrams (mg) of brivaracetam.
The other components are: sodium citrate, anhydrous citric acid, methyl parahydroxybenzoate (E218), sodium carmellose, sucralose, liquid sorbitol (E420), glycerol (E422), raspberry flavor (propylene glycol (E1520) 90% - 98%), purified water.
Appearance of the Product and Package Contents
Briviact 10 mg/ml oral solution is a slightly viscous, transparent, colorless to yellowish liquid.
The 300 ml glass bottle of Briviact is packaged in a cardboard box with a 10 ml oral syringe (black graduation marks), a 5 ml oral syringe (blue graduation marks), and adapters for the syringes.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
UCB Pharma, S.A., Allée de la Recherche 60, B-1070, Brussels, Belgium.
Manufacturer
UCB Pharma, S.A., Chemin du Foriest, B-1420 Braine-l’Alleud, Belgium.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium UCB Pharma SA/NV Tel: + 32 / (0)2 559 92 00 | Lithuania UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Finland) |
Bulgaria UCB Pharma Bulgaria EOOD Tel: + 359 (0) 2 962 30 49 | Luxembourg UCB Pharma SA/NV Tel: + 32 / (0)2 559 92 00 (Belgium) |
Czech Republic UCB s.r.o. Tel: + 420 221 773 411 | Hungary UCB Magyarország Kft. Tel: + 36-(1) 391 0060 |
Denmark UCB Nordic A/S Tel: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Germany UCB Pharma GmbH Tel: + 49 /(0) 2173 48 4848 | Netherlands UCB Pharma B.V. Tel: + 31 / (0)76-573 11 40 |
Estonia UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Finland) | Norway UCB Nordic A/S Tel: + 47 / 67 16 5880 |
Greece UCB Α.Ε. Tel: + 30 / 2109974000 | Austria UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
Spain UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Poland UCB Pharma Sp. z o.o. / VEDIM Sp. z o.o. Tel: + 48 22 696 99 20 |
France UCB Pharma S.A. Tel: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 / 21 302 5300 |
Croatia Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | Romania UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Slovenia Medis, d.o.o. Tel: + 386 1 589 69 00 |
Iceland Vistor hf. Tel: + 354 535 7000 | Slovakia UCB s.r.o., organizational unit Tel: + 421 (0) 2 5920 2020 |
Italy UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Finland UCB Pharma Oy Finland Tel: + 358 9 2514 4221 |
Cyprus Lifepharma (Z.A.M.) Ltd Tel: + 357 22 05 63 00 | Sweden UCB Nordic A/S Tel: + 46 / (0) 40 29 49 00 |
Latvia UCB Pharma OY Finland Tel: + 358 9 2514 4221 (Finland) | United Kingdom(Northern Ireland) UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Average pharmacy price119.89 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRIVIACT 10 mg/ml ORAL SOLUTIONDosage form: TABLET, 100 mgActive substance: brivaracetamManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 25 mgActive substance: brivaracetamManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 50 mgActive substance: brivaracetamManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for BRIVIACT 10 mg/ml ORAL SOLUTION
Discuss questions about BRIVIACT 10 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








