
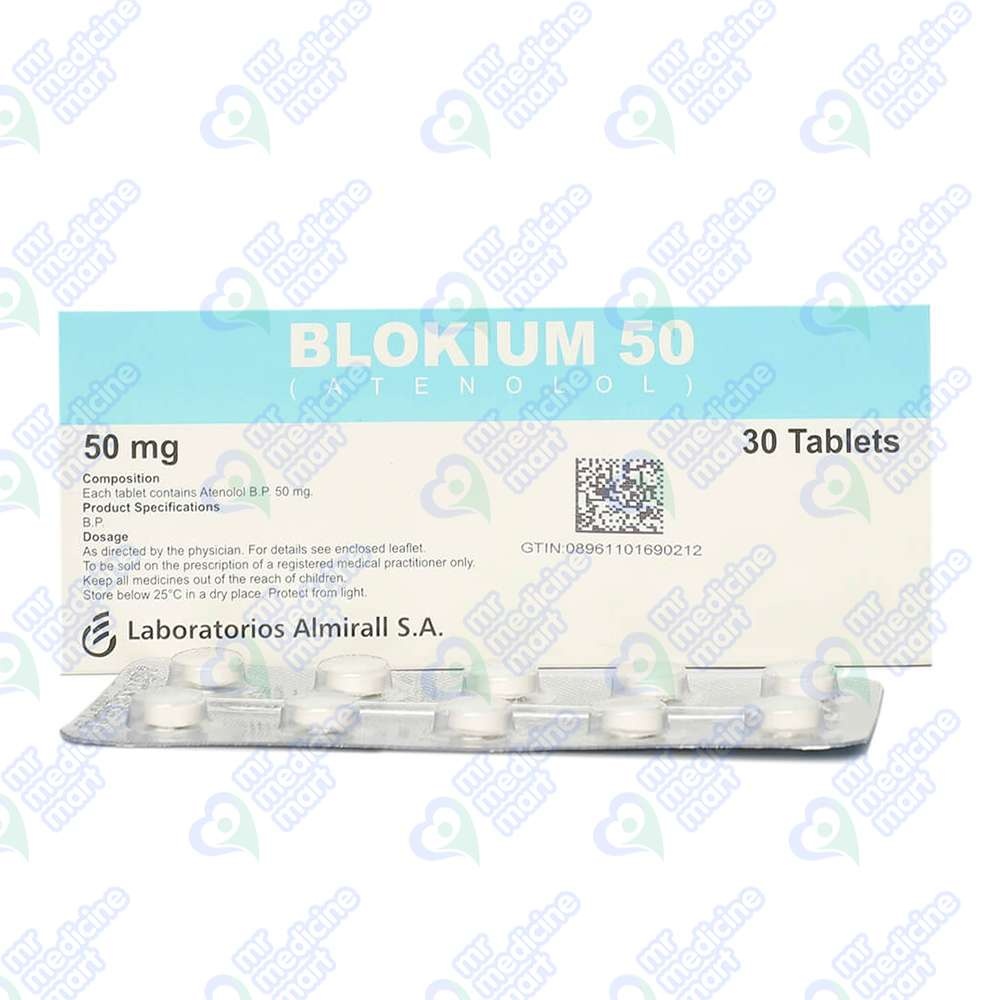
BLOKIUM 50 mg TABLETS

How to use BLOKIUM 50 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Blokium 50 mg Tablets
atenolol
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the Package Leaflet
- What is Blokium 50 mg and what is it used for
- What you need to know before you take Blokium 50 mg
- How to take Blokium 50 mg
- Possible side effects
- Storage of Blokium 50 mg
- Contents of the pack and further information
1. What is Blokium 50 mg and what is it used for
Blokium 50 mg belongs to a group of medicines called beta-blockers, which act mainly on the heart.
Doctors prescribe this medicine to people with high blood pressure (hypertension), to prevent chest pain of coronary origin (angina), and to regulate heartbeats in arrhythmias. It also protects the heart during and after a heart attack (myocardial infarction).
2. What you need to know before you take Blokium 50 mg
Do not takeBlokium 50 mg
- if you are allergic to atenolol or any of the other ingredients of this medicine (listed in section 6).
- if you have or have had heart diseases such as uncontrolled heart failure (the heart does not pump enough blood to the rest of the body), bradycardia (very slow pulse), second- or third-degree heart block, or sick sinus syndrome (diseases in which the pulse is irregular).
- if you have ever had very low blood pressure, circulatory failure, or cardiac shock.
- if you have been told that you have a pheochromocytoma and are not being treated, or if you have metabolic acidosis.
Warnings and precautions
Consult your doctor before starting to take this medicine.
- if you have health problems such as asthma or breathing difficulties, if you have diabetes, depression, circulatory disorders, heart problems other than those mentioned in the previous section, kidney, liver, or thyroid problems.
- if you have been diagnosed with a type of chest pain called Prinzmetal's angina.
- if you have ever had an allergic reaction to something, for example, an insect bite, you should be cautious when taking this medicine, as it may increase the severity of these allergic reactions.
- if you notice that your pulse is slower while taking these tablets. This is normal, but if you are concerned, inform your doctor.
- if you are diabetic, this medicine may mask a drop in blood sugar (hypoglycemia) by reducing the normal response, which is an increased heart rate. It may also mask the manifestations of hyperthyroidism or thyrotoxicosis (disorders in which thyroid hormone levels are elevated in the blood).
- in case you are hospitalized, inform the healthcare staff and, in particular, the anesthesiologist, that you are being treated with this medicine, as certain medicines used in anesthesia may be incompatible with Blokium.
If you have any doubts about any of the above points, consult your doctor before taking Blokium.
If you are going to have any diagnostic tests (including blood tests, urine tests, skin tests that use allergens, etc.), inform your doctor that you are taking this medicine, as it may alter the results. See section 4.
Other medicines and Blokium 50 mg
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Be especially careful and inform your doctor if you are taking disopyramide (for irregular heartbeats), other treatments for hypertension or angina (in particular, verapamil, diltiazem, nifedipine, clonidine), treatment for heart failure (digoxin), indomethacin or ibuprofen (for inflammation and/or pain), adrenaline (used in case of cardiac arrest or severe allergic reactions), or reserpine (for hypertension).
The administration of other beta-blockers such as celiprolol, propranolol, metoprolol, timolol, bisoprolol, carvedilol, oxprenolol, or nebivolol may increase the depressant effect of atenolol (the active ingredient of this medicine) on the heart.
You should also inform your doctor if you are being treated with any medicine for nasal congestion or other cold products that you may have purchased yourself at a pharmacy.
If you are taking clonidine for hypertension or to prevent migraine, do not stop treatment with this or Blokium without consulting your doctor first.
Never take another medicine on your own initiative without your doctor's recommendation, as some combinations should be avoided.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The use of this medicine is not recommended during breastfeeding; if your doctor considers that you must take it, it is recommended to replace breastfeeding with formula feeding.
Children and adolescents
This medicine should not be used in children.
Use in people over 65 years old
Elderly patients have a higher risk of suffering adverse reactions due to the use of this medicine, so it should be used with caution in these patients (see section 3).
Use in athletes
Athletes are informed that this medicine contains a component that may produce a positive result in doping tests.
Driving and using machines
Some people may occasionally experience dizziness and fatigue while being treated with this medicine. If you feel these effects, you should not drive vehicles or operate machinery.
This medicine contains less than 23 mg of sodium (1 mmol) per tablet; it is essentially "sodium-free".
3. How to take Blokium 50 mg
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Remember to take your medicine.
This medicine is for oral administration. Swallow the tablet whole with the help of water.
Try to take your tablet at the same time every day.
Your doctor will decide the dose of this medicine that you should take based on your individual needs. The usual doses are:
Hypertension:the usual dose is 100 mg, in a single daily intake. Some patients may need a maintenance dose of only 50 mg, once a day. The evaluation of the response should only be done after 1 or 2 weeks of continuous treatment.
In case the reduction of tension, after this time, is insufficient, your doctor will assess the need to add a diuretic or another antihypertensive to the treatment.
Angina pectoris:the effective dose is generally 100 mg in a single dose or in two doses of 50 mg per day. It is not usual for the efficacy to increase if this dose is exceeded.
Arrhythmias:after initial treatment with atenolol intravenously, maintenance therapy with 50 or 100 mg of atenolol, orally, in a single daily dose, may be started.
Acute myocardial infarction:it is possible that during the early phase of a myocardial infarction, you will be administered a dose of atenolol intravenously after the onset of chest pain. Subsequently, if no adverse effects have appeared, you should take this medicine orally as indicated. The dose after this acute phase will be 100 mg per day orally. If adverse effects such as a significant decrease in pulse and/or blood pressure appear, treatment will be discontinued.
Use in people over 65 years old
Your doctor should adjust the dose of this medicine if you are over 65 years old.
Use in patients with kidney disease
Your doctor should adjust the dose of this medicine if you have kidney disease.
Use in patients with liver disease
Your doctor should adjust the dose of this medicine if you have liver disease.
If you think the action of this medicine is too strong or too weak, tell your doctor or pharmacist.
If you take more Blokium 50 mg than you should
You may have difficulty breathing or a decrease in pulse or blood pressure. Contact your doctor or go to the nearest hospital as soon as possible. Take this leaflet with you.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Telephone 915 620 420.
If you forget to take Blokium 50 mg
If you forget to take this medicine, take a dose as soon as you remember. Do not take a double dose to make up for forgotten doses.
If you stop taking Blokium 50 mg
Your doctor will indicate the duration of your treatment with this medicine. Do not stop taking the tablets before finishing the treatment, even if you feel well, unless your doctor tells you to. If your doctor tells you to stop taking this medicine, you should stop treatment gradually.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Blokium 50 mg can cause side effects, although not everybody gets them.
If any of the following side effects occur, inform your doctor, who will indicate whether you should interrupt the treatment and how to do it correctly.
The side effects that have been observed with this medicine are the following:
- Very common (affecting more than 1 in 10 patients): fatigue, drowsiness, headache, insomnia, depression, difficulty breathing (bronchospasm), circulation problems, with cold extremities and a feeling of tingling.
- Common (affecting between 1 and 10 in 100 patients): severe circulatory disorders (decrease in blood pressure and/or pulse, heart block, heart failure), hallucinations, somnolence, confusion, neurological disorders such as paresthesia (tingling and numbness of the extremities) or peripheral neuropathy (pain, loss of sensitivity, and inability to control muscles), muscle disorders, and vision disorders.
- Uncommon (affecting between 1 and 10 in 1,000 patients): diarrhea, constipation, nausea, vomiting, abdominal cramps (abdominal pain), allergic reactions, skin rashes, itching, reversible hair loss, decreased platelets, decreased white blood cells, non-thrombocytopenic purpura (a blood disorder characterized by bruising and bleeding), increased eosinophils in blood, impotence (erectile dysfunction), decreased libido, pulmonary fibrosis, and pleural effusion.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Blokium 50 mg
Do not store above 30°C.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE  collection point at the pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
collection point at the pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Blokium 50 mg
- The active ingredient is atenolol. Each tablet contains 50 mg of atenolol.
- The other ingredients are microcrystalline cellulose (E-460), magnesium stearate (E-572), sodium starch glycolate (from potato), povidone.
Appearance of the product and pack contents
Blokium 50 mg tablets are white, round, flat, with a central score line on the upper face and a smooth lower face.
Blokium 50 mg tablets are available in packs containing 30 or 60 tablets.
Marketing authorization holder and manufacturer
Marketing authorization holder
Almirall, S.A.
General Mitre, 151
08022 Barcelona (Spain)
Manufacturer
Industrias Farmacéuticas Almirall, S.A.
Ctra. de Martorell, 41-61
08740 Sant Andreu de la Barca - Barcelona (Spain)
This leaflet was approved in:December 2002
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price2.5 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BLOKIUM 50 mg TABLETSDosage form: TABLET, 100 mgActive substance: atenololManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 50 mgActive substance: atenololManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 100 mgActive substance: atenololManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for BLOKIUM 50 mg TABLETS
Discuss questions about BLOKIUM 50 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











