
BIMZELX 320 mg Injectable Solution in Pre-filled Pen

How to use BIMZELX 320 mg Injectable Solution in Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Bimzelx320mg solution for injection in pre-filled pen
bimekizumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Bimzelx and what is it used for
- What you need to know before you use Bimzelx
- How to use Bimzelx
- Possible side effects
- Storage of Bimzelx
- Contents of the pack and other information
Instructions for use
1. What is Bimzelx and what is it used for
What is Bimzelx
Bimzelx contains the active substance bimekizumab.
What is Bimzelx used for
Bimzelx is used to treat the following inflammatory diseases:
- Plaque psoriasis
- Psoriatic arthritis
- Axial spondyloarthritis, including non-radiographic axial spondyloarthritis and ankylosing spondylitis (radiographic axial spondyloarthritis)
- Hidradenitis suppurativa
Plaque psoriasis
Bimzelx is used in adults to treat a skin condition called plaque psoriasis. Bimzelx reduces symptoms, including pain, itching, and skin scaling.
Psoriatic arthritis
Bimzelx is used to treat adults with psoriatic arthritis. Psoriatic arthritis is a disease that causes joint inflammation, often accompanied by plaque psoriasis. If you have active psoriatic arthritis, you may have been given other medicines before. If these medicines do not work well enough or if you cannot tolerate them, you will receive Bimzelx alone or in combination with another medicine called methotrexate.
Bimzelx reduces inflammation and can help reduce pain, stiffness, swelling in the joints and around them, psoriatic skin rash, and nail damage, as well as slow down cartilage and bone damage in the affected joints. These effects help control the signs and symptoms of the disease, make it easier to perform daily activities, reduce fatigue, and improve quality of life.
Axial spondyloarthritis, including non-radiographic axial spondyloarthritis and ankylosing spondylitis (radiographic axial spondyloarthritis)
Bimzelx is used to treat adults with a disease that mainly affects the spine and causes inflammation of the joints in the spine, called axial spondyloarthritis. If the condition is not visible on X-rays, it is called "non-radiographic axial spondyloarthritis"; if it occurs in patients with visible signs on X-rays, it is called "ankylosing spondylitis" or "radiographic axial spondyloarthritis".
If you have axial spondyloarthritis, you will first be given other medicines. If you do not respond well enough to these medicines, you will be given Bimzelx to reduce the signs and symptoms of the disease, decrease inflammation, and improve your physical functional ability. Bimzelx can help reduce back pain, stiffness, and fatigue, making it easier to perform daily activities and improve quality of life.
Hidradenitis suppurativa
Bimzelx is used in adults to treat a disease called hidradenitis suppurativa (sometimes referred to as inverse acne or Verneuil's disease). Hidradenitis suppurativa is a chronic inflammatory skin disease that causes painful lesions in the form of nodules (lumps) and abscesses (boils), as well as lesions that can ooze pus. It mainly affects specific areas of the skin, such as under the breasts, armpits, inner thighs, groin, and buttocks. Scars can also form in the affected areas. You will first be given other medicines. If your response to these medicines is not sufficient, you will be given Bimzelx.
Bimzelx reduces inflammatory nodules (lumps), abscesses (boils), and lesions that can ooze pus, as well as pain caused by hidradenitis suppurativa.
How Bimzelx works
Bimekizumab, the active substance in Bimzelx, belongs to a group of medicines called interleukin inhibitors (IL). Bimekizumab works by reducing the activity of two interleukins called IL-17A and IL-17F, which are involved in the production of inflammation. The levels of these interleukins are elevated in inflammatory diseases such as psoriasis, psoriatic arthritis, axial spondyloarthritis, and hidradenitis suppurativa.
2. What you need to know before you use Bimzelx
Do not use Bimzelx
- If you are allergic to bimekizumab or any of the other ingredients of this medicine (listed in section 6).
- If you have an infection that your doctor considers important, such as tuberculosis (TB).
Warnings and precautions
Tell your doctor, pharmacist, or nurse before starting treatment with Bimzelx if:
- You have an infection or an infection that keeps coming back.
- You have been vaccinated recently or are planning to be vaccinated. During treatment with Bimzelx, some vaccines (those made with live microbes) should not be given.
- You have had tuberculosis (TB) in the past.
- You have had inflammatory bowel disease (Crohn's disease or ulcerative colitis) in the past.
Inflammatory bowel disease (Crohn's disease or ulcerative colitis)
Stop using Bimzelx and tell your doctor or seek medical attention immediately if you notice blood in your stools, abdominal cramps, pain, diarrhea, or weight loss. These may be signs of the onset or worsening of inflammatory bowel disease (Crohn's disease or ulcerative colitis).
Be aware of the risk of infections and allergic reactions
Rarely, Bimzelx can cause serious infections. Talk to your doctor or seek medical attention immediately if you notice any signs of serious infection. These signs are listed in "Serious side effects" in section 4.
Bimzelx can cause serious allergic reactions. Talk to your doctor or seek medical attention immediately if you notice any signs of a serious allergic reaction. These signs may include:
- difficulty breathing or swallowing
- low blood pressure, which can cause dizziness or fainting
- swelling of the face, lips, tongue, or throat
- severe itching of the skin, with a red rash or bumps
Children and adolescents
This medicine must not be used in children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and Bimzelx
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. It is recommended to avoid using Bimzelx during pregnancy, as it is not known how this medicine will affect the baby.
If you are a woman who can become pregnant, you must use contraception while using this medicine and for at least 17 weeks after the last dose of Bimzelx.
If you are breastfeeding or plan to breastfeed, consult your doctor before using this medicine. You and your doctor will decide whether you can breastfeed or use Bimzelx.
Driving and using machines
Bimzelx is unlikely to affect your ability to drive or use machines.
Bimzelx containspolysorbate 80
This medicine contains 0.4 mg of polysorbate 80 in each ml of solution. Polysorbates can cause allergic reactions. Tell your doctor if you have any known allergies.
Bimzelx contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which is essentially "sodium-free".
3. How to use Bimzelx
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
How much Bimzelx to use and for how long
Plaque psoriasis
The recommended dose, given as an injection under the skin ("subcutaneous injection"), is as follows:
- 320 mg (given in onepre-filled pen with 320 mg) at weeks 0, 4, 8, 12, and 16.
- From week 16, you will use 320 mg (onepre-filled pen with 320 mg) every 8 weeks. If you weigh more than 120 kg, your doctor may decide to continue with injections every 4 weeks from week 16.
Psoriatic arthritis
The recommended dose, given as an injection under the skin ("subcutaneous injection"), is as follows:
- 160 mg every 4 weeks.
- If you have co-existing psoriatic arthritis with moderate to severe plaque psoriasis, the recommended dosing regimen is the same as for plaque psoriasis. After week 16, your doctor may adjust the injections to 160 mg every 4 weeks, based on your joint symptoms. Other pharmaceutical forms/concentrations are available for administration of the 160 mg dose.
Axial spondyloarthritis, including non-radiographic axial spondyloarthritis and ankylosing spondylitis (radiographic axial spondyloarthritis)
The recommended dose, given as an injection under the skin ("subcutaneous injection"), is as follows:
- 160 mg every 4 weeks. Other pharmaceutical forms/concentrations are available for administration of the 160 mg dose.
Hidradenitis suppurativa
The recommended dose, given as an injection under the skin ("subcutaneous injection"), is as follows:
- 320 mg (given in onepre-filled pen with 320 mg) every 2 weeks until week 16.
- From week 16, you will receive 320 mg (onepre-filled pen with 320 mg each) every 4 weeks.
You and your doctor or nurse will decide if you should inject this medicine yourself. Do not inject this medicine unless a healthcare professional has taught you how to do it. A caregiver can also give you the injections if they have been taught how to do it.
Read the "Instructions for use" at the end of this leaflet before injecting the pre-filled pen of Bimzelx 320 mg solution for injection yourself.
If you use more Bimzelx than you should
Tell your doctor if you have used more Bimzelx than you should or if you have injected a dose too early.
If you forget to use Bimzelx
Talk to your doctor if you have forgotten to inject a dose of Bimzelx.
If you stop using Bimzelx
Talk to your doctor before stopping treatment with Bimzelx. If you stop treatment, your symptoms may return.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Tell your doctor or seek medical attention immediately if you experience any of the following side effects:
Possible serious infection - the signs may include:
- fever, flu-like symptoms, night sweats
- feeling tired or having trouble breathing, persistent cough
- heat, redness, and pain in the skin or a painful rash with blisters
Your doctor will decide if you can continue using Bimzelx.
Other side effects
Tell your doctor, pharmacist, or nurse if you experience any of the following side effects:
Very common (may affect more than 1 in 10 people)
- upper respiratory tract infections with symptoms such as sore throat and nasal congestion
Common (may affect up to 1 in 10 people)
- oral candidiasis with symptoms such as yellow or white patches; redness or sores in the mouth and pain when swallowing
- fungal infection of the skin, such as athlete's foot between the toes
- ear infections
- fever (herpes simplex infections)
- stomach flu (gastroenteritis)
- inflammation of the hair follicles, which can look like pimples
- headache
- itching, dry skin, or a rash similar to eczema, sometimes with swollen and red skin
- acne
- redness, pain, or swelling and bruising at the injection site
- feeling tired
- fungal infection of the vulvovaginal area (vaginal candidiasis)
Uncommon (may affect up to 1 in 100 people)
- low levels of white blood cells (neutropenia)
- fungal infections of the skin and mucous membranes (including esophageal candidiasis)
- discharge in the eye with itching, redness, and swelling (conjunctivitis)
- blood in the stools, abdominal cramps, pain, diarrhea, or weight loss (signs of intestinal problems)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Bimzelx
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP/CAD. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C to 8°C). Do not freeze.
Keep the pre-filled pens in the original carton to protect them from light.
Bimzelx can be stored at room temperature (up to 25°C) for a maximum of 25 days. It must be kept in the outer packaging and away from direct light. Do not use the pre-filled pens after this time. There is a space on the carton for you to write the date you removed it from the refrigerator.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Bimzelx Composition
- The active substance is bimekizumab. Each pre-filled pen contains 320 mg of bimekizumab in 2 ml of solution.
- The other components are glycine, sodium acetate trihydrate, glacial acetic acid, polysorbate 80, and water for injectable preparations.
Appearance of Bimzelx and Container Contents
Bimzelx is a clear to slightly opalescent liquid. The color may vary from colorless to light brown-yellow. It comes in a single-use, disposable pre-filled pen.
Bimzelx 320 mg injectable solution in a pre-filled syringe is available in packs containing 1 pre-filled pen and in multipacks of 3 boxes, each containing 1 pre-filled pen.
Only some pack sizes may be marketed.
Marketing Authorization Holder
UCB Pharma S.A.
Allée de la Recherche 60
B-1070 Brussels, Belgium
Manufacturer
UCB Pharma S.A.
Chemin du Foriest
B-1420 Braine-l’Alleud, Belgium
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien UCB Pharma S.A./NV Tel: + 32 / (0)2 559 92 00 | Lithuania UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Finland) |
Bulgaria UCB Pharma Tel: + 359 (0) 2 962 30 49 | Luxembourg/Luxemburg UCB Pharma S.A./NV Tel: + 32 / (0)2 559 92 00 (Belgium/Belgien) |
Czech Republic UCB s.r.o. Tel: + 420 221 773 411 | Hungary UCB Hungary Kft. Tel: + 36-(1) 391 0060 |
Denmark UCB Nordic A/S Tel: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Germany UCB Pharma GmbH Tel: + 49 / (0)2173 48 4848 | Netherlands UCB Pharma B.V. Tel: + 31 / (0)76-573 11 40 |
Estonia UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Finland) | Norway UCB Nordic A/S Tel: + 47 / 67 16 5880 |
Greece UCB Α.Ε. Tel: + 30 / 2109974000 | Austria UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
Spain UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Poland UCB Pharma Sp. z o.o./VEDIM Sp. z o.o. Tel: + 48 22 696 99 20 |
France UCB Pharma S.A. Tel: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 21 302 5300 |
Croatia Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Romania UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 Slovenia Medis, d.o.o. Tel: + 386 1 589 69 00 |
Iceland Vistor hf. Tel: + 354 535 7000 | Slovakia UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Italy UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Finland UCB Pharma Oy Finland Tel: + 358 9 2514 4221 |
Cyprus Lifepharma (Z.A.M.) Ltd Tel: + 357 22 056300 | Sweden UCB Nordic A/S Tel: + 46 / (0) 40 294 900 |
Latvia UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Finland) | |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu
Instructions for Use
Read the following instructions in their entirety before using the Bimzelx 320 mg pre-filled pen.
Bimzelx 320 mg pre-filled pen at a glance (see Figure A):
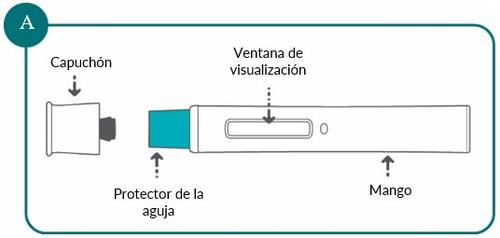
Important Information:
- Your healthcare professional should teach you how to prepare and inject Bimzelx using the 320 mg pre-filled pen. Do notinject yourself or someone else until you have been taught how to inject Bimzelx correctly.
- You and/or your caregiver should read these instructions before each use of Bimzelx.
- Call your healthcare professional if you or your caregiver have any questions about how to inject Bimzelx correctly.
- Each pre-filled pen is for single use (single dose) only.
Do not use this medicine and return it to the pharmacy if:
- The expiration date (EXP/CAD) has passed.
- The pack is damaged.
- The pre-filled pen has been dropped or appears to be damaged.
- The liquid has been frozen at any time (even if it has been thawed).
For a more comfortable injection:Remove the 320 mg pre-filled pen from the refrigerator and let it sit on a flat surface at room temperature in its original packaging for 30-45 minutesbefore injection.
- Do not heat it in any other way, such as in a microwave or with hot water.
- Do not shake the pre-filled pen.
- Do not remove the cap from the pre-filled pen until you are ready to inject.
Follow the steps below each time you use Bimzelx.
Step 1: Prepare the Injection
Place the following items on a clean, flat, and well-lit work surface, such as a table:
- 1 Bimzelx 320 mg pre-filled pen
You will also need (not included in the box):
- 1 alcohol swab
- 1 clean cotton ball
- 1 sharps disposal container. See “Dispose of the used Bimzelx pre-filled pen” at the end of these instructions for use.
Step 2: Choose the Injection Site and Prepare the Injection
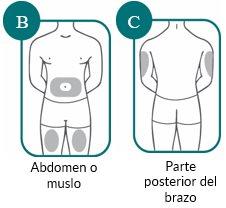
2a: Choose the Injection Site
- The injection sites you can choose from are:
- The stomach (abdomen) or thigh (see Figure B);
- The back of the arm can also be used if a caregiver is administering the injection (see Figure C).
- Do not inject into areas where the skin is sensitive, bruised, red, scaly, or hard, or into areas with scars or stretch marks.
- Do not inject within 5 cm of the navel.
- You must choose a different injection site each time you administer an injection.
2b: Wash your hands well with water and soap and dry them with a dry and clean towel
2c: Prepare the skin
- Clean the injection site with an alcohol swab. Let the area dry completely. Do not touch the cleaned area before injection.
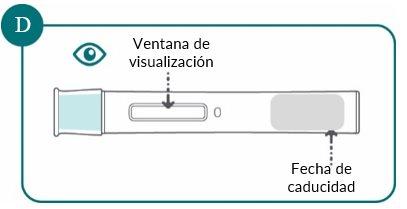
2d: Check the pre-filled pen (see Figure D)
- Make sure the name Bimzelx and the expiration date appear on the label.
- Check the medicine through the viewing window. The medicine should be clear to slightly opalescent and free of particles. The color may vary from colorless to light brown-yellow. You may see air bubbles in the liquid. This is normal.
- Do not use the Bimzelx pre-filled pen if the medicine is cloudy, has changed color, or contains particles.


Step 3: Inject Bimzelx
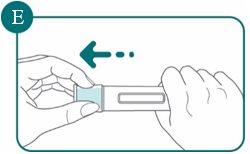
3a: Remove the cap from the pre-filled pen
- Hold the pre-filled pen firmly with one hand by the handle. Pull the cap straight off the pre-filled pen with the other hand (see Figure E). Although you cannot see the needle tip, it is now exposed.
- Do not touch the needle shield or replace the cap. This is because you could activate the pre-filled pen and stick yourself.
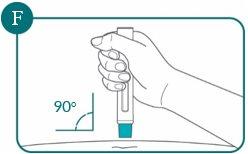
3b: Hold the pre-filled pen at a 90-degree angle to the cleaned injection site (see Figure F)
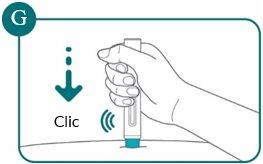
3c: Place the pre-filled pen flat against the skin and then press the pre-filled pen firmly against the skin
You will hear a click. The injection starts when you hear the first “click” (see Figure G).
Do not lift the pre-filled pen away from the skin.
3d: Hold the pre-filled pen in place and press it firmly against the skin. It will take approximately 20 seconds to receive the full dose.
- You will hear a second “click” that indicates the injection is almost complete. You will see the yellow indicator filling the viewing window (see Figure H).
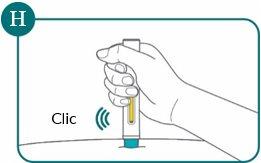
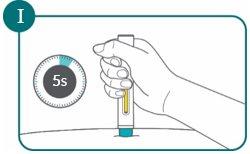
- After the second click, continue to press the pre-filled pen for another 5 seconds (count slowly to 5). This will ensure you receive the full dose (see Figure I).

3e: Remove the pre-filled pen by carefully pulling it straight out of the skin. The needle shield will automatically cover the needle
- Press a dry cotton ball against the injection site for a few seconds. Do not rub the injection site. You may see a small amount of bleeding or a drop of liquid. This is normal. You can cover the injection site with a bandage if needed.
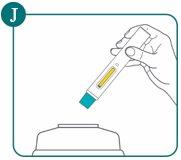
Step 4: Dispose of the used Bimzelx pre-filled pen
Place the used pre-filled pen in a sharps disposal container immediately after use (see Figure J).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BIMZELX 320 mg Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 160 mgActive substance: bimekizumabManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE, 160 mgActive substance: bimekizumabManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for BIMZELX 320 mg Injectable Solution in Pre-filled Pen
Discuss questions about BIMZELX 320 mg Injectable Solution in Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






