
BELOKEN 1 mg/ml INJECTABLE SOLUTION

How to use BELOKEN 1 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Beloken 1 mg/ml Solution for Injection
metoprolol tartrate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Beloken 1 mg/ml and what is it used for
- What you need to know before you are given Beloken 1 mg/ml
- How Beloken 1 mg/ml will be given to you
- Possible side effects
- Storing Beloken 1 mg/ml
Contents of the pack and further information
1. What is Beloken 1 mg/ml and what is it used for
The active substance is metoprolol tartrate, which belongs to a group of medicines called beta-blockers. These medicines reduce the effect of stress hormones on some parts of the body. In this way, metoprolol is able to act on blood vessels and the heart, reducing blood pressure and heart rate.
This medicine is used in adults for the treatment of:
- Heart rhythm disorders, especially rapid heartbeats (supraventricular tachycardia).
- Treatment of patients who are hemodynamically stable with acute myocardial infarction defined or suspected. (Early intervention in the acute phase, prior to oral therapy).
2. What you need to know before you are given Beloken 1 mg/ml
Do not use Beloken 1 mg/ml
- if you are allergic to metoprolol or any of the other ingredients of this medicine (listed in section 6).
- if you have unstable heart failure, blockage (heart conduction disorder) or acute myocardial infarction.
- if you have ever had very slow or irregular heartbeats or circulatory failure.
The use of Beloken 1 mg/ml is not recommended in children.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Beloken:
- if you have signs/symptoms of low blood glucose levels (hypoglycemia).
- if you have health problems such as asthma or breathing difficulties, circulatory disorders, heart problems, kidney or thyroid problems.
- if during treatment with this medicine your heart rate slows down more and more.
- if you have ever been told that you have pheochromocytoma (a tumor in the adrenal glands).
- if you have very low blood pressure or severely impaired blood circulation.
- if you have uncontrolled heart failure.
- if you are allergic to insect bites, foods, or other substances.
Tell your doctor about any health problems you have had in the past.
If you are an athlete, note that this medicine contains a component that may result in a positive doping test.
If you have not informed your doctor about any of the above situations or have any doubts, consult your doctor or pharmacist before starting treatment with this medicine.
Using Beloken 1 mg/ml with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Some medicines may affect the action of other medicines.
In particular, inform your doctor if you are using any of the following medicines, as you may need to adjust the dose of one of the medicines:
- Medicines used in heart and blood vessel treatments (such as digitalis/digoxin, calcium antagonists (verapamil or diltiazem), anti-arrhythmic agents (quinidine or amiodarone), ganglion-blocking agents, hydralazine, clonidine).
- Other medicines such as monoamine oxidase inhibitors (MAOIs), inhaled anesthetics, antibiotics (rifampicin), anti-ulcer medicines (cimetidine), non-steroidal anti-inflammatory drugs (e.g., indomethacin, celecoxib), certain antidepressants and antipsychotics, antihistamines, other beta-blockers (e.g., eye drops, asthma medications), and other substances (e.g., alcohol, certain hormones, such as adrenaline), oral antidiabetics.
Consider that:
- If you take clonidine and Beloken 1 mg/ml together and need to stop treatment with clonidine, you should stop taking metoprolol several days before stopping clonidine.
- If you are taking oral antidiabetics, your doctor may need to adjust the dose.
- The blood levels of this medicine may be increased if administered with anti-arrhythmic agents, antihistamines, antidepressants, antipsychotics, non-steroidal anti-inflammatory drugs, alcohol, or hydralazine.
- The blood levels of this medicine may be decreased by rifampicin.
- Administration of this medicine with anti-arrhythmic agents (quinidine or amiodarone) results in an increased effect of these medicines.
- The effects of this medicine may be increased when administered with calcium antagonists (verapamil or diltiazem). Concomitant administration of these medicines should be avoided.
- The effects of this medicine may be decreased when administered with adrenaline or indomethacin.
Taking Beloken 1 mg/ml with alcohol
Consuming alcohol may increase the levels of metoprolol in the blood, increasing the effect of the medicine. Avoid drinking alcohol with this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before you are given this medicine.
Beta-blockers, including metoprolol, may harm the fetus and cause premature birth.
Tell your doctor if you are breastfeeding, as Beloken 1 mg/ml may cause adverse effects in the breastfed baby, such as a slow heart rate.
Driving and using machines
Some patients may feel tired or dizzy when taking Beloken. If this happens, do not drive or operate tools or machines.
Beloken 1 mg/ml contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per 5 ml of solution for injection (one ampoule); this is essentially "sodium-free".
3. How Beloken 1 mg/ml will be given to you
Metoprolol intravenous injection is used in acute situations where rapid onset of action is necessary and should only be administered by expert personnel who can provide more information.
Your doctor will tell you how long you will need to be treated with this medicine. Do not stop treatment unless your doctor tells you to.
Treatment of patients who are hemodynamically stable with acute myocardial infarction defined or suspected. (Early intervention in the acute phase, prior to oral therapy):In the acute phase and as soon as possible after the patient arrives at the hospital, 5 mg of metoprolol will be administered intravenously as a bolus. A second and third injection of 5 mg will be repeated at 2-minute intervals, depending on the patient's hemodynamic status. (See "Before using Beloken 1 mg/ml").
Patients who have tolerated the total intravenous dose of 15 mg will be given 50 mg of metoprolol succinate or tartrate tablets 4 times a day for 48 hours, starting 15 minutes after the last injection.
Patients who do not tolerate the total intravenous dose of 15 mg will start oral treatment with caution, beginning with a lower dose.
Heart rhythm disorders, especially rapid heartbeats (supraventricular tachycardia):Initially up to 5 mg administered intravenously at a rate of 1-2 mg/minute. This dose may be repeated at 5-minute intervals until a satisfactory effect is achieved. A total dose of 10-15 mg usually achieves this effect.
Doses of 20 mg or more are unnecessary, as they do not provide greater therapeutic benefit.
Use in children and adolescents
It is not recommended due to limited experience with Beloken in children and adolescents.
Use in elderly patients
The dose of this medicine does not need to be adjusted in elderly patients.
If you think the action of this medicine is too strong or too weak, tell your doctor, healthcare professional, or pharmacist.
If you are given too much Beloken 1 mg/ml
If you think you have been given too much Beloken 1 mg/ml, consult your doctor or pharmacist immediately.
In case of using a dose of this medicine higher than recommended, if it is high enough, you may suffer from poisoning with some of the following symptoms: slow or irregular heartbeat, difficulty breathing, swelling of the ankles, feeling of palpitations, fainting, dizziness, chest pain, cold skin, weak pulse, mental confusion, anxiety, cardiac arrest, loss of consciousness (or even coma), nausea, vomiting, blue discoloration of the skin, or low blood pressure (hypotension).
The first signs of overdose may appear between 20 minutes and 2 hours after administration of the medicine. If you experience any of these symptoms, contact your doctor, pharmacist, or the nearest hospital immediately.
If you take alcohol, antihypertensive drugs, quinidine, or sleeping pills (barbiturics) with metoprolol, the symptoms may worsen.
Treatment:
In case of severe hypotension and shock, plasma or plasma substitutes will be administered, and the patient will be kept under observation in the intensive care unit.
If the heart rate is excessively slow or irregular, atropine may be used intravenously and/or a pacemaker. If necessary, glucagon and/or dobutamine may also be administered, and the administration of calcium ions may be considered.
Bronchospasm can be reversed with a bronchodilator. For more information, see the Summary of Product Characteristics.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Beloken 1 mg/ml is well-tolerated, and the undesirable effects that may occur are generally mild and disappear when treatment is stopped.
The following side effects have been reported in patients treated with metoprolol, although the relationship with metoprolol treatment has not been established in all cases. If you experience any of the following reactions persistently, tell your doctor.
Very common(may affect more than 1 in 10 people):
- fatigue.
Common(may affect up to 1 in 10 people):
- dizziness, headache,
- slow heart rate, dizziness when changing position (very rarely with loss of consciousness), cold hands and feet, palpitations,
- nausea, abdominal pain, diarrhea, constipation,
- shortness of breath when exerting oneself.
Uncommon (may affect up to 1 in 100 people):
- feeling of warmth/pins and needles/numbness, muscle cramps,
- symptoms of heart disease such as shortness of breath, weakness, and swelling of the ankles may worsen temporarily,
- during a heart attack, blood pressure may drop excessively (cardiogenic shock), minor changes in the electrocardiogram without affecting heart function, swelling, chest pain,
- depression, altered concentration, drowsiness, lack of sleep, nightmares,
- skin rash, increased sweating,
- feeling of pressure in the airways,
- vomiting,
- weight gain.
Rare (may affect up to 1 in 1,000 people):
- changes in heart conduction in the electrocardiogram, irregular heartbeats,
- nervousness, anxiety,
- liver problems (abnormalities in liver function tests),
- hair loss,
- runny nose due to allergic reaction,
- vision problems, dryness and/or irritation of the eyes,
- dry mouth, tearing, or irritation of the eyes due to allergic reaction,
- impotence/sexual dysfunction.
Very rare (may affect up to 1 in 10,000 people):
- worsening of circulatory problems in the limbs in patients with severe circulatory disorders,
- joint pain,
- loss or impairment of memory, confusion, hallucinations,
- skin reaction due to increased sensitivity to sunlight, worsening of psoriasis,
- ringing in the ears,
- changes in taste,
- blood disorders (decrease in the number of platelets in the blood)
- hepatitis.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines and Healthcare Products Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Beloken 1 mg/ml
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C.
Store the ampoules in the original package until use.
6. Contents of the pack and further information
Composition of Beloken 1 mg/ml
- The active substance is metoprolol tartrate 5 mg.
- The other ingredients (excipients) are: sodium chloride, water for injection, c.s.p. 5 ml.
Appearance and packaging of the product
Beloken 1 mg/ml is presented in a pack of 5 glass ampoules of 5 mg (1 mg/ml).
Marketing authorization holder and manufacturer
Marketing authorization holder:
Recordati Industria Chimica e Farmaceutica S.p.A.
Via Matteo Civitali, 1
20148 Milano
Italy
Manufacturer:
Cenexi
52 Rue Marcel et Jacques Gaucher
94120 Fontenay Sous Bois
France
or
CIT S.r.l.
Via Primo Villa, 17
20875 Burago di Molgora (MB)
Italy
You can request more information about this medicine from the local representative of the marketing authorization holder:
Casen Recordati, S.L.
Autovía de Logroño, km 13,300
50180 Utebo - Zaragoza
Spain
Date of last revision of this leaflet: January 2021
This information is intended only for healthcare professionals:
METHOD OF ADMINISTRATION
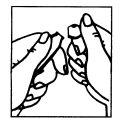
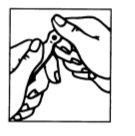
Figure 1 Figure 2
- The ampoule has a mark below the blue point (Figure 1).
- Place your thumb on the blue point and break the ampoule at the neck (Figure 2).
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BELOKEN 1 mg/ml INJECTABLE SOLUTIONDosage form: TABLET, 100 mgActive substance: metoprololManufacturer: Casen Recordati S.L.Prescription requiredDosage form: TABLET, 100 mgActive substance: metoprololManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 100 mgActive substance: atenololManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for BELOKEN 1 mg/ml INJECTABLE SOLUTION
Discuss questions about BELOKEN 1 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











