
ATARAX 2 mg/ml SYRUP

How to use ATARAX 2 mg/ml SYRUP
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Atarax 2mg/ml syrup
hydroxyzine, dihydrochloride
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Atarax syrup is and what it is used for
- What you need to know before you take Atarax syrup
- How to take Atarax syrup
- Possible side effects
- Storage of Atarax syrup
- Contents of the pack and further information
1. What Atarax syrup is and what it is used for
Atarax is an anxiolytic medicine that belongs to a class of compounds called diphenylmethanes.
Atarax is used in the:
- symptomatic treatment of anxiety in adults over 18 years of age;
- symptomatic treatment of pruritus in adults, adolescents, and children from 12 months of age.
2. What you need to know before you take Atarax syrup
Do not take Atarax
- If you are allergic (hypersensitive) to hydroxyzine dihydrochloride or any of the other ingredients of Atarax, to cetirizine, to other piperazine derivatives, to aminophylline, or to ethylenediamine.
- If you have porphyria (a group of inherited disorders that affect the blood).
- If your ECG (electrocardiogram) shows a heart rhythm problem called "prolonged QT interval".
- If you have or have had a cardiovascular disease or if your heart rate is very low.
- If you have low levels of salts in your body (e.g., low potassium or magnesium levels).
- If you are taking certain medicines for heart rhythm problems or medicines that may affect the heart rhythm (see "Using Atarax with other medicines").
- If a close relative has died suddenly due to heart problems.
- In case of pregnancy or breastfeeding.
Warnings and precautions
- If you have a high risk of seizures.
- If you have liver failure and if you have moderate or severe kidney failure. In these cases, the dose of Atarax should be reduced.
- If you suffer from glaucoma, obstruction of the flow of the urinary bladder, decreased gastrointestinal motility, myasthenia gravis, or dementia.
- Atarax may be associated with an increased risk of heart rhythm disorders that can be life-threatening. Therefore, inform your doctor if you have any heart problems or if you are taking any other medicine, including those purchased without a prescription.
Seek medical attention immediately if, while being treated with Atarax, you experience heart problems such as palpitations, difficulty breathing, or loss of consciousness. Treatment with hydroxyzine should be discontinued.
Other medicines and Atarax
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes any medicines purchased without a prescription. Atarax may affect or be affected by other medicines.
Atarax may enhance the depressant effect on the central nervous system if used in conjunction with other drugs with depressant or anticholinergic properties. In these cases, the dose should be adjusted for each patient.
Atarax antagonizes the effects of betahistine and anticholinesterase drugs.
Treatment with Atarax should be suspended at least 5 days before performing an allergy test or a bronchial provocation test with methacholine, to avoid effects on the results.
The simultaneous administration of Atarax with monoamine oxidase inhibitors should be avoided.
Atarax counteracts the pressor effect of adrenaline.
The concomitant administration with drugs that may cause arrhythmias may increase the risk of QT prolongation and Torsades de Pointes (alterations in electrocardiogram measurements).
Do not take Atarax if you are taking medicines to treat:
- bacterial infections (e.g., the antibiotics erythromycin, moxifloxacin, levofloxacin)
- fungal infections (e.g., pentamidine)
- heart problems or high blood pressure (e.g., amiodarone, quinidine, disopyramide, sotalol)
- psychosis (e.g., haloperidol)
- depression (e.g., citalopram, escitalopram)
- gastrointestinal disorders (e.g., prucalopride)
- allergy
- malaria (e.g., mefloquine and hydroxychloroquine)
- cancer (e.g., toremifene, vandetanib)
- drug abuse or severe pain (methadone)
To date, no further interactions with other medicines have been detected.
Taking Atarax with food, drinks, and alcohol
Do not drink alcohol while being treated with this medicine. The simultaneous administration of hydroxyzine and alcohol may produce effects on the central nervous system.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before taking any medicine. Atarax should not be taken during pregnancy.
Atarax should not be taken during breastfeeding. If treatment with Atarax is necessary, breastfeeding should be discontinued.
The following reactions may occur in newborns of mothers who have taken Atarax during the last stage of pregnancy and/or childbirth, which can be observed immediately or a few hours after birth: tremors, stiffness and/or muscle weakness, difficulty breathing, and urinary retention (urine retention).
Driving and using machines
Atarax may alter your ability to drive or operate machinery, as it may cause drowsiness, decrease your attention, or decrease your reaction ability. The appearance of these effects is more likely at the start of treatment or when the dose is increased. Do not drive or use machines if you experience any of these effects.
Elderly patients
The use of this medicine is not recommended in elderly patients, as in these patients the effect of the medicine may be prolonged and the risk of side effects may increase. If it is necessary to use this medicine in elderly patients, it is recommended to start treatment with half the recommended dose.
Atarax contains sodium benzoate, sucrose, and ethanol
This medicine contains less than 1 mmol of sodium (23 mg) per 5 ml; this is, essentially "sodium-free".
This medicine contains 1.5 mg of sodium benzoate in each 5 ml, equivalent to 0.3 mg/ml.
This medicine contains sucrose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine. Sucrose may harm teeth. At doses greater than 6.5 ml of syrup, the sucrose content should be taken into account in patients with diabetes mellitus.
This medicine contains 4.75 mg of alcohol (ethanol) in each 5 ml, which is equivalent to 0.95 mg/ml (0.095% p/v). The amount in 5 ml of this medicine is equivalent to less than 2 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect.
3. How to take Atarax syrup
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
The smallest dose of Atarax that is effective should be administered and for the shortest possible time.
Adults over 18 years of age:
- For the symptomatic treatment of anxiety: 50 mg (25 ml) per day, divided into 3 doses. It is recommended to administer 12.5 mg (6.25 ml) in the morning, 12.5 mg (6.25 ml) at noon, and 25 mg (12.5 ml) at night. Your doctor will indicate if you need to take a higher dose at night. In more severe cases, up to 100 mg (50 ml) per day can be used. The maximum daily dose is 100 mg (50 ml) per day.
- For the symptomatic treatment of pruritus: It is recommended to start with a dose of 25 mg (12.5 ml) one hour before bedtime, and if necessary, administer doses of up to 25 mg (12.5 ml) 3 to 4 times a day. The maximum daily dose is 100 mg (50 ml) per day.
Adolescents and children from 12 months of age:
- For the symptomatic treatment of pruritus: 1 to 2 mg (0.5 to 1 ml) per kg per day, divided into several doses. In children up to 40 kg in weight, the maximum daily dose is 2 mg (1 ml) per kg per day. In children over 40 kg in weight, the maximum daily dose is 100 mg (50 ml) per day.
The use of this medicine is not recommended in elderly patients (see section 2). If it is necessary to use this medicine, it is recommended to start treatment with half the recommended dose. Your doctor will prescribe the smallest possible dose. The maximum daily dose in these patients is 50 mg (25 ml) per day.
If you have kidney or liver problems, your doctor may indicate that you take a lower dose (see "Warnings and precautions").
The dose will be adjusted within the recommended dose range according to the patient's response to treatment.
If you think the effect of Atarax is too strong or too weak, consult your doctor or pharmacist.
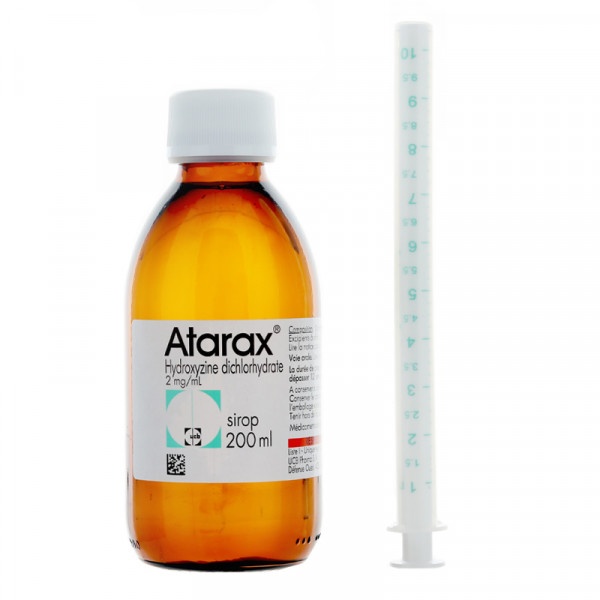
The amount of syrup is measured with a 10 ml oral syringe, graduated every 0.25 ml. Each ml contains 2 mg of hydroxyzine dihydrochloride. Insert the syringe included in the package into the bottle and extract the corresponding ml.
The syrup should be taken before meals, alone or diluted in water or fruit juice.
If you take more Atarax than you should
If you have taken too much Atarax, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, especially if it is a child who has taken too much. In case of overdose, symptomatic treatment can be established. Electrocardiographic monitoring (ECG) can be performed due to the possibility of a heart rhythm problem, such as QT interval prolongation or Torsades de Pointes.
A significant overdose can cause nausea, vomiting, tachycardia, fever, drowsiness, altered pupillary reflex, tremor, confusion, hallucination, decreased level of consciousness, respiratory depression, convulsions, decreased blood pressure, and cardiac arrhythmia, including bradycardia, which can lead to a deep coma and cardiopulmonary collapse.
If you forget to take Atarax
Remember to always take your medicine.
Do not take a double dose to make up for forgotten doses. Continue taking your normal dose when it is due.
If you stop taking Atarax
Your doctor will indicate the duration of your treatment with Atarax. Do not stop treatment before.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported in clinical trials:
- drowsiness, headache, fatigue, dry mouth.
The frequent side effect (which affects at least 1 in 100 patients) is sedation.
The uncommon side effects (which affect at least 1 in 1,000 patients) are: nausea, discomfort, fever, dizziness, insomnia, tremor, agitation, and confusion.
The rare side effects (which affect at least 1 in 10,000 patients) are: tachycardia, alterations in accommodation or ability to see at different distances, blurred vision, constipation, vomiting, hypersensitivity, abnormal liver function tests, convulsions, involuntary abnormal movements (dyskinesia), disorientation, hallucinations, urinary retention, erythematous rash, maculopapular rash, dermatitis, itching, and hypotension.
The very rare side effects (which affect less than 1 in 10,000 patients) are: anaphylactic shock, bronchospasm (narrowing of the bronchi that causes difficulty breathing), angioneurotic edema (inflammation of the skin and mucous membranes), increased sweating, drug rash, acute generalized exanthematous pustulosis (a type of allergic reaction that appears in response to a medicine, infection, or disease), erythema multiforme, Stevens-Johnson syndrome (these two are skin disorders due to an allergic reaction or infection).
The side effects of unknown frequency (which cannot be estimated from the available data) are: QT prolongation in the electrocardiogram, Torsades de Pointes (alteration of electrocardiogram measurements), hepatitis, loss of consciousness (syncope), blistering diseases (e.g., toxic epidermal necrolysis, pemphigus), and weight gain.
Stop taking this medicine and seek medical attention immediately if you experience any heart rhythm problems such as palpitations, difficulty breathing, or loss of consciousness.
The following side effects have been observed with cetirizine, the main metabolite of hydroxyzine: thrombocytopenia (decrease in the number of platelets), aggression, depression, tic, dystonia (muscle contractions), paresthesia (tingling sensation), oculogyric crisis (fixation of the eyes in a fixed position), diarrhea, dysuria (difficulty urinating), enuresis (urinary incontinence), asthenia, edema, and weight gain.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Atarax syrup
Keep this medicine out of the sight and reach of children.
Atarax should be stored in its carton box because the active ingredient hydroxyzine dihydrochloride is sensitive to light.
No special storage temperature is required.
Do not use Atarax syrup after the expiration date stated on the packaging after "EXP". The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines that you no longer need in the SIGRE collection point at the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines that you no longer need.
In this way, you will help protect the environment.
6. Contents of the pack and further information
Composition of Atarax syrup
- The active ingredient is hydroxyzine dihydrochloride. Each ml contains 2 mg of hydroxyzine dihydrochloride.
- The other ingredients are: sucrose, sodium benzoate, hazelnut flavor, levomenthol, ethanol, purified water.
Appearance of the product and pack contents
Atarax syrup is presented in a topaz glass bottle with a child-resistant cap and an oral syringe (Polyethylene/Polyester) of 10 ml, graduated every 0.25 ml. Each pack contains 150 ml of syrup.
Marketing authorization holder and manufacturer
Marketing authorization holder:
UCB Pharma, S.A.
Plaza de Manuel Gómez Moreno, s/n,
Edificio Bronce, Planta 5,
28020 Madrid
Spain
Manufacturer:
NEXTPHARMA, SAS
17, Route de Meulan (Limay)
78250 - France
Date of the last revision of this leaflet:January 2023
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price1.92 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATARAX 2 mg/ml SYRUPDosage form: TABLET, 25 mg hydroxyzine dihydrochlorideActive substance: hydroxyzineManufacturer: Ucb Pharma S.A.Prescription requiredDosage form: TABLET, 25 mgActive substance: hydroxyzineManufacturer: Bluefish Pharmaceuticals Ab (Publ)Prescription requiredDosage form: TABLET, 25 mgActive substance: hydroxyzineManufacturer: Neuraxpharm Spain S.L.Prescription required
Online doctors for ATARAX 2 mg/ml SYRUP
Discuss questions about ATARAX 2 mg/ml SYRUP, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












