
ARANESP 300 micrograms INJECTABLE SOLUTION IN PRE-FILLED SYRINGE

How to use ARANESP 300 micrograms INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Aranesp 10 micrograms solution for injection in pre-filled syringe
Aranesp 15 micrograms solution for injection in pre-filled syringe
Aranesp 20 micrograms solution for injection in pre-filled syringe
Aranesp 30 micrograms solution for injection in pre-filled syringe
Aranesp 40 micrograms solution for injection in pre-filled syringe
Aranesp 50 micrograms solution for injection in pre-filled syringe
Aranesp 60 micrograms solution for injection in pre-filled syringe
Aranesp 80 micrograms solution for injection in pre-filled syringe
Aranesp 100 micrograms solution for injection in pre-filled syringe
Aranesp 130 micrograms solution for injection in pre-filled syringe
Aranesp 150 micrograms solution for injection in pre-filled syringe
Aranesp 300 micrograms solution for injection in pre-filled syringe
Aranesp 500 micrograms solution for injection in pre-filled syringe
darbepoetin alfa (darbepoetin alfa)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Aranesp and what is it used for
- What you need to know before you use Aranesp
- How to use Aranesp
- Possible side effects
- Storing Aranesp
- Contents of the pack and other information
1. What is Aranesp and what is it used for
Your doctor has prescribed Aranesp (an anti-anaemic) for the treatment of your anaemia. Anaemia occurs when the blood does not contain enough red blood cells and the symptoms can be tiredness, weakness and shortness of breath.
Aranesp works in exactly the same way as the natural hormone erythropoietin. Erythropoietin is produced in the kidneys and helps the bone marrow to produce more red blood cells. The active substance of Aranesp is darbepoetin alfa, produced by genetic engineering in Chinese Hamster Ovary (CHO-K1) cells.
If you have chronic kidney disease
Aranesp is used to treat symptomatic anaemia associated with chronic kidney disease (kidney failure) in adults and children. In kidney failure, the kidney does not produce enough of the natural hormone erythropoietin, which can often cause anaemia.
As your body will need some time to produce more red blood cells, it will take about four weeks before you notice any effect. Your normal dialysis routine will not affect Aranesp's ability to treat your anaemia.
If you are receiving chemotherapy
Aranesp is used to treat symptomatic anaemia in adult patients with non-myeloid tumours receiving chemotherapy.
One of the main side effects of chemotherapy is that it stops the bone marrow from producing enough red blood cells. Towards the end of chemotherapy treatment, especially if you have received a lot of chemotherapy, your red blood cell count may decrease, causing anaemia.
2. What you need to know before you use Aranesp
Do not use Aranesp:
- if you are allergic to darbepoetin alfa or any of the other ingredients of this medicine (listed in section 6).
- if you have high blood pressure that is not being treated with other medicines prescribed by your doctor.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you start using Aranesp.
Tell your doctor if you have or have had:
- high blood pressure that is being treated with medicines prescribed by your doctor;
- sickle cell anaemia;
- epileptic seizures (fits);
- convulsions (fits and seizures);
- liver disease;
- if you do not respond to medicines used to treat anaemia;
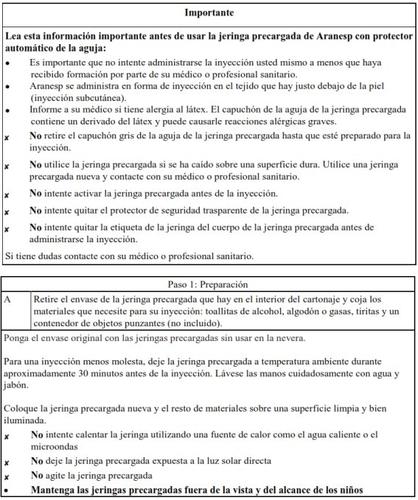
- allergy to latex (the needle cap of the pre-filled syringe contains a latex derivative); or
- hepatitis C.
Special precautions:
- If you have symptoms such as more tiredness than usual and lack of energy, it may be due to a disorder called pure red cell aplasia (PRCA) that has been observed in some patients. PRCA means that the body has reduced or stopped the production of red blood cells, causing severe anaemia. If you experience these symptoms, contact your doctor, who will determine the best way to treat your anaemia.
- Be cautious with other medicines that stimulate the production of red blood cells: Aranesp belongs to a group of medicines that stimulate the production of red blood cells, such as human erythropoietic proteins. Your doctor should always keep a record of the exact medicine you are using.
- If you are a patient with chronic kidney disease and especially if you do not respond correctly to Aranesp, your doctor will review the dose of Aranesp, as if you do not respond to treatment, repeated increases in the dose of Aranesp could increase the risk of having a heart or blood vessel problem and could increase the risk of heart attack, stroke and death.
- Your doctor will try to keep your haemoglobin levels between 10 and 12 g/dl. Your doctor will check that your haemoglobin does not exceed a certain level, as high concentrations of haemoglobin could put you at risk of having a heart or blood vessel problem and could increase the risk of heart attack, stroke and death.
- If you experience symptoms that include severe headache, numbness, confusion, vision problems, nausea, vomiting or seizures (convulsions), it may mean that you have very high blood pressure. If you experience these symptoms, you should contact your doctor.
- If you are a cancer patient, you should know that Aranesp may act as a growth factor for blood cells and that in some circumstances it may have a negative effect on your cancer. Depending on your individual situation, it may be preferable to have a blood transfusion. Please discuss this with your doctor.
- The use of this medicine in healthy subjects can cause heart or blood vessel problems that can be fatal.
- Severe skin reactions associated with epoetin treatment, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported. SJS/TEN can appear initially as red spots similar to a target or circular spots often with central blisters on the torso. In addition, there may be ulcers in the mouth, throat, nose, genitals and eyes (red and swollen eyes). These severe rashes are often preceded by fever or flu-like symptoms. The rashes can progress to widespread skin peeling and potentially fatal complications. If you experience a severe rash or any of these skin symptoms, stop taking Aranesp and inform your doctor or seek immediate medical attention.
Using Aranesp with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Ciclosporin and tacrolimus (medicines that suppress the immune system) may be affected by the number of red blood cells in the blood. It is important that you tell your doctor if you are using any of these medicines.
Using Aranesp with food and drink
Food and drink do not affect Aranesp.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Aranesp has not been used in pregnant women. It is important that you tell your doctor if you:
- are pregnant;
- think you may be pregnant; or
- are planning to have a baby.
It is not known whether darbepoetin alfa is excreted in breast milk. If you are treated with Aranesp, you should stop breast-feeding.
Driving and using machines
Aranesp should not affect your ability to drive or use machines.
Aranesp contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, i.e. it is essentially "sodium-free".
3. How to use Aranesp
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
After some blood tests, your doctor has decided that you need Aranesp because your haemoglobin level is 10 g/dl or less. Your doctor will tell you how much Aranesp you need and how often you should use it to keep your haemoglobin level between 10 and 12 g/dl. This may vary depending on whether you are an adult or a child.
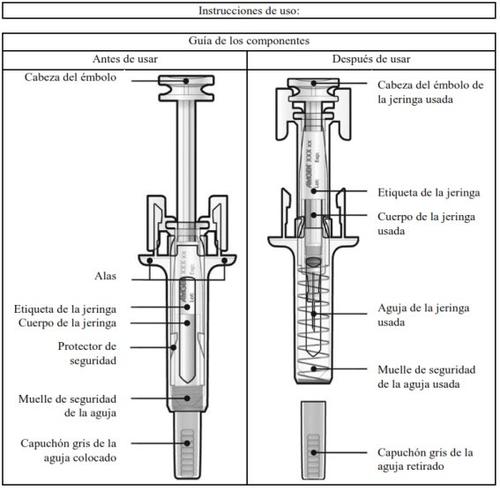
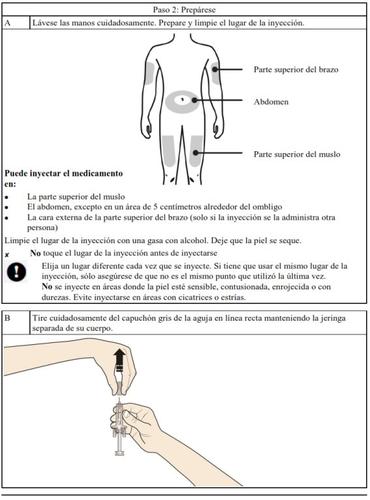
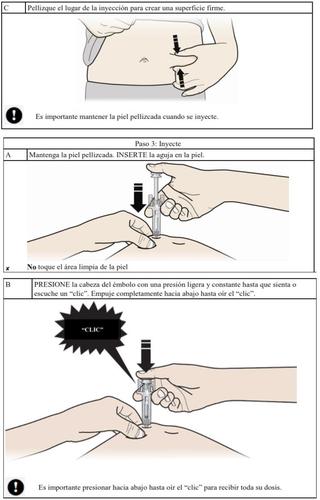
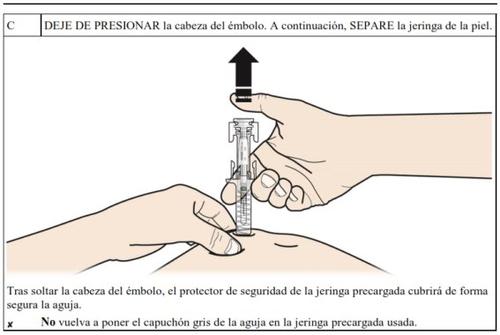
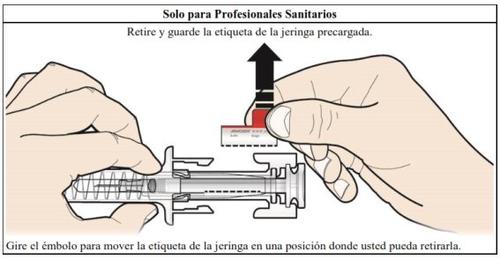
How to inject Aranesp yourself
Your doctor may decide that it is better for you or the people taking care of you to inject Aranesp. Your doctor, nurse or pharmacist will teach you how to inject the pre-filled syringe. Do not try to inject yourself if you have not been taught how to do it. Never inject Aranesp yourself into a vein.
If you have chronic kidney disease
For all adult and paediatric patients ≥ 1 year of age with chronic kidney disease, Aranesp is administered in a single injection, under the skin (subcutaneously) or in a vein (intravenously).
To correct anaemia, the initial dose of Aranesp per kilogram of body weight will be:
- 0.75 micrograms once every two weeks, or
- 0.45 micrograms once a week.
For adult patients not on dialysis, 1.5 micrograms/kg once a month may also be used as the initial dose.
For all adult and paediatric patients ≥ 1 year of age with chronic kidney disease, once anaemia is corrected, you will continue to receive Aranesp in a single injection, either once a week or once every two weeks. For all adult and paediatric patients ≥ 11 years of age who are not on dialysis, Aranesp may also be administered as a monthly injection.
Your doctor will regularly take blood samples to see how your anaemia is responding to treatment and, if necessary, may need to adjust the dose every four weeks to maintain long-term control of your anaemia.
Your doctor will use the lowest effective dose to control the symptoms of your anaemia.
If you do not respond adequately to Aranesp, your doctor will review your dose and inform you if you need to change the doses of Aranesp.
Your doctor will regularly check your blood pressure, especially at the start of treatment.
In some cases, your doctor may recommend that you take iron supplements.
Your doctor may decide to change the way you are given the injection (under the skin or in a vein). If this happens, you will start with the same dose you were receiving before and you will have blood tests to check that your anaemia is still being treated correctly.
If your doctor has decided to change your treatment from r-HuEPO (genetically engineered erythropoietin) to Aranesp, they will choose to administer Aranesp once a week or once every two weeks. The route of administration will be the same as with r-HuEPO, but your doctor will decide how much and when you should be given it, and may adjust the dose you receive if necessary.
If you are receiving chemotherapy
Aranesp is administered under the skin in a single injection, once a week or once every three weeks.
To correct anaemia, the initial dose of Aranesp will be:
- 500 micrograms once every three weeks (6.75 micrograms of Aranesp per kilogram of body weight), or
- 2.25 micrograms of Aranesp per kilogram of body weight (once a week).
Your doctor will regularly take blood samples to measure how your anaemia is responding, and may adjust the dose as necessary. Treatment will continue until approximately 4 weeks after the end of chemotherapy. Your doctor will tell you exactly when to stop taking Aranesp.
In some cases, your doctor may recommend that you take iron supplements.
If you use more Aranesp than you should
You may have serious problems if you inject more Aranesp than you should, such as very high blood pressure. Contact your doctor or pharmacist if this happens. If you do not feel well, contact your doctor or pharmacist immediately.
If you forget to use Aranesp
Do not take a double dose to make up for forgotten doses.
If you forget to inject a dose of Aranesp, contact your doctor to see when you should inject the next dose.
If you stop using Aranesp
If you want to stop using Aranesp, you should first discuss it with your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some patients have experienced the following side effects using Aranesp:
Patients with chronic kidney disease
Very common:may affect more than 1 in 10 people
- High blood pressure (hypertension)
- Allergic reactions
Common:may affect up to 1 in 10 people
- Stroke
- Pain at the injection site
- Rash and/or redness of the skin
Uncommon:may affect up to 1 in 100 people
- Blood clots (thrombosis)
- Convulsions (fits and seizures)
Frequency not known:frequency cannot be estimated from the available data
- Pure red cell aplasia (PRCA) - (anaemia, more tiredness than usual, lack of energy)
Patients with cancer
Very common:may affect more than 1 in 10 people
- Allergic reactions
- Fluid retention (oedema)
Common:may affect up to 1 in 10 people
- High blood pressure (hypertension)
- Blood clots (thrombosis)
- Pain at the injection site
- Rash and/or redness of the skin
Uncommon:may affect up to 1 in 100 people
- Convulsions (fits and seizures)
All patients
Frequency not known:frequency cannot be estimated from the available data
- Severe allergic reactions that can include:
- Life-threatening allergic reactions (anaphylaxis)
- Swelling of the face, lips, mouth, tongue or throat that can cause difficulty swallowing or breathing (angioedema)
- Breathing difficulties (allergic bronchospasm)
- Skin rashes
- Hives (urticaria)
- Severe skin reactions including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in association with epoetin treatment. These can appear as red spots similar to a target or circular spots often with central blisters on the torso, skin peeling, ulcers in the mouth, throat, nose, genitals and eyes and can be preceded by fever or flu-like symptoms.
Stop taking Aranesp if you experience these symptoms and inform your doctor or seek immediate medical attention. See also section 2.
- Bruising and bleeding at the injection site
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Aranesp
Keep this medicine out of the sight and reach of children.
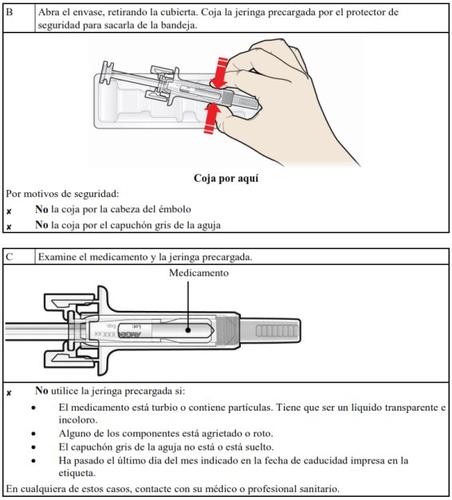
Do not use this medicine after the expiry date which is stated on the carton and on the label after “EXP”. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C - 8°C). Do not freeze. Do not use Aranesp if you think it has been frozen.
Keep the pre-filled syringe in the outer packaging to protect it from light.
Once you have removed your syringe from the refrigerator and left it at room temperature for about 30 minutes before injecting it, you must use it within 7 days or discard it.
Do not use this medicine if you notice that the contents of the syringe are cloudy or contain particles.
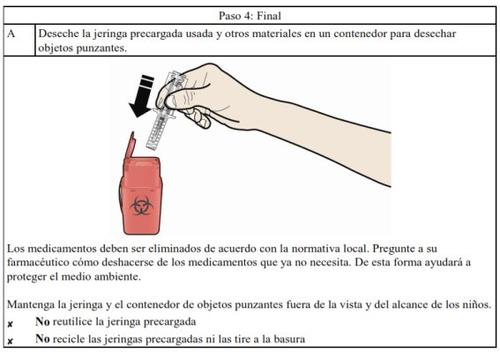
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Package Contents and Additional Information
Aranesp Composition
- The active ingredient is darbepoetina alfa, r-HuEPO (erythropoietin produced by genetic technology). The pre-filled syringes contain 10, 15, 20, 30, 40, 50, 60, 80, 100, 130, 150, 300 or 500 micrograms of darbepoetina alfa.
- The other components are sodium phosphate monobasic, sodium phosphate dibasic, sodium chloride, polysorbate 80, and water for injectables.
Product Appearance and Package Contents
Aranesp is a clear, colorless or slightly opalescent injectable solution in a pre-filled syringe.
Aranesp is available in packs of 1 or 4 pre-filled syringes with an automatic needle protector, packaged in a blister pack. Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Amgen Europe B.V.
Minervum 7061
NL-4817 ZK Breda
Netherlands
Marketing Authorization Holder
Amgen Europe B.V.
Minervum 7061
NL-4817 ZK Breda
Netherlands
Manufacturer
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
For further information about this medicinal product, please contact the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien s.a. Amgen n.v. Tel/Tél: +32 (0)2 7752711 | Lithuania Amgen Switzerland AG Vilnius branch Tel: +370 5 219 7474 |
Bulgaria Amgen Bulgaria EOOD Tel: +359 (0)2 424 7440 | Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tel/Tél: +32 (0)2 7752711 |
Czech Republic Amgen s.r.o. Tel: +420 221 773 500 | Hungary Amgen Kft. Tel: +36 1 35 44 700 |
Denmark Amgen, branch of Amgen AB, Sweden Tlf: +45 39617500 | Malta Amgen B.V. The Netherlands Tel: +31 (0)76 5732500 |
Germany AMGEN GmbH Tel: +49 89 1490960 | Netherlands Amgen B.V. Tel: +31 (0)76 5732500 |
Estonia Amgen Switzerland AG Vilnius branch Tel: +372 586 09553 | Norway Amgen AB Tel: +47 23308000 |
Greece Amgen Hellas Pharmaceuticals S.A. Tel: +30 210 3447000 | Austria Amgen GmbH Tel: +43 (0)1 50 217 |
Spain Amgen S.A. Tel: +34 93 600 18 60 | Poland Amgen Biotechnology Sp. z o.o. Tel: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220550 |
Croatia Amgen d.o.o. Tel: +385 (0)1 562 57 20 | Romania Amgen Romania SRL Tel: +4021 527 3000 |
Ireland Amgen Limited United Kingdom Tel: +44 (0)1223 420305 | Slovenia AMGEN d.o.o. Tel: +386 (0)1 585 1767 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italy Amgen S.r.l. Tel: +39 02 6241121 | Finland Amgen AB, branch in Finland Tel: +358 (0)9 54900500 |
Cyprus C.A. Papaellinas Ltd Tel: +357 22741 741 | Sweden Amgen AB Tel: +46 (0)8 6951100 |
Latvia Amgen Switzerland AG Riga branch Tel: +371 257 25888 | United Kingdom Amgen Limited Tel: +44 (0)1223 420305 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu.
This leaflet is available in all EU/EEA languages on the European Medicines Agency website.

- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ARANESP 300 micrograms INJECTABLE SOLUTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, UnknownActive substance: darbepoetin alfaManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: darbepoetin alfaManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 100 µgActive substance: darbepoetin alfaManufacturer: Amgen Europe B.V.Prescription required
Online doctors for ARANESP 300 micrograms INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Discuss questions about ARANESP 300 micrograms INJECTABLE SOLUTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















