APRETUDE 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION

How to use APRETUDE 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Apretude 600 mg prolonged-release injectable suspension
cabotegravir
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can contribute by reporting any side effects you may have. The last part of section 4 will include information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Apretude and what is it used for
- What you need to know before you are given Apretude
- How Apretude is given
- Possible side effects
- Storage of Apretude
- Contents of the pack and other information
1. What is Apretude and what is it used for
Apretude contains cabotegravir as the active substance. Cabotegravir belongs to a group of antiretroviral medicines called integrase inhibitors (INI).
Apretude is used to help prevent infection with HIV-1 in adults and adolescents who weigh at least 35 kg and are at high risk of infection. This is called pre-exposure prophylaxis: PrEP(see section 2).
It must be used in combination with safer sex practices, such as using condoms.
2. What you need to know before you are given Apretude
Do not use Apretude
- if you have ever developed a severe skin rash, skin peeling, blisters, and/or sores in the mouth.
- if you are allergic(hypersensitive) to cabotegravir or any of the other ingredients of this medicine (listed in section 6).
- If you are HIV positiveor you do not know if you are HIV positive. Apretude can only help reduce the risk of getting HIV before you become infected. You must get testedto make sure you are HIV negative before taking Apretude.
- if you are taking any of the following medicines:
- carbamazepine, oxcarbazepine, phenytoin, phenobarbital(medicines to treat epilepsy and prevent seizures).
- rifampicin or rifapentine(medicines to treat some bacterial infections, such as tuberculosis).
These medicines reduce the effectiveness of Apretude by decreasing the amount of Apretude in your blood.
If you think this applies to you, or if you are not sure, tell your doctor.
Warnings and precautions
Using Apretude on its own may not prevent HIV infection.
HIV infection is spread by sexual contact with someone who is HIV positive or by transfer of infected blood. Although Apretude reduces the risk of infection, you can still get HIV even if you are taking this medicine.
You must take other measures to reduce your risk of getting HIV:
- Get testedfor other sexually transmitted diseases when your doctor tells you to. These infections make it easier for you to get HIV.
- Use condomswhen you have oral or penetrative sex.
- Do not share or reuse needles or other injection equipment or medical equipment.
- Do not share personal items that may contain blood or body fluids (such as razors or toothbrushes).
Talk to your doctor about what other precautions you need to take to reduce your risk of getting HIV.
Reduce your risk of getting HIV:
There is a risk of developing resistance to this medicine if you get HIV. This means the medicine may not prevent you from getting HIV. To minimize this risk and prevent HIV infection, it is essential that you:
- attend your scheduled appointmentsto receive your Apretude injection. Tell your doctor if you are thinking of stopping treatment, as this may increase your risk of getting HIV. If you stop getting your Apretude injection or delay it, you may need to take other medicines or precautions to reduce your risk of getting HIV and possibly develop viral resistance.
- get tested for HIVwhen your doctor tells you to. You should get tested regularly to make sure you are still HIV-1 negative while taking Apretude.
- tell your doctor immediatelyif you think you may have gotten HIV (you may have flu-like symptoms). You may need to get more tests to make sure you are still HIV negative.
Apretude injectable is a long-acting medicine
If you stop getting Apretude injections, cabotegravir may stay in your body for more than a year after your last injection, but this will not be enough to protect you from possible infection.
It is essential that you attend your scheduled appointments to receive your Apretude injections. Talk to your doctor if you are thinking of stopping PrEP.
Once you stop Apretude injectable, you may need to take other medicines to reduce your risk of getting HIV or use other precautions like safe sex.
Liver problems
Tell your doctor if you have liver problems. You may need to be monitored more closely. (See also ‘Uncommon side effects’ in section 4).
Adolescents
Your doctor will talk to you about your mental health before and while you are taking Apretude. Tell your doctor if you have mental health problems. You may need to be monitored more closely (See also section 4).
Severe skin reaction
Very rare cases of severe skin reactions, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported with Apretude. If you notice any of the symptoms related to these severe skin reactions, do not receive your next Apretude injection and seek medical attention immediately.
Read the informationin section 4 of this leaflet (“Possible side effects”).
Allergic reactions
Apretude contains cabotegravir, which is an integrase inhibitor. Integrase inhibitors, including cabotegravir, can cause a severe allergic reaction known as a hypersensitivity reaction. You need to know what the important signs and symptoms are to look out for while taking Apretude.
Read the informationin ‘Possible side effects’ in section 4 of this leaflet.
Children and adolescents
This medicine must not be used in children and adolescents who weigh less than 35 kg, as it has not been studied in these users.
Other medicines and Apretude
Tell your doctor if you are taking, have recently taken, or might take any other medicines, including medicines bought without a prescription.
Some medicines may affect how Apretude works or increase your chance of side effects. Apretude may also affect how other medicines work.
Apretude must not be givenwith other medicines that may affect the effectiveness of the medicine (see ‘Do not use Apretude’ in section 2). These include:
- carbamazepine, oxcarbazepine, phenytoin, phenobarbital(medicines to treat epilepsy and prevent seizures).
- rifampicin or rifapentine(medicines to treat some bacterial infections, such as tuberculosis).
Tell your doctorif you are taking:
- rifabutin(to treat some bacterial infections, such as tuberculosis). You may need to receive Apretude injections more frequently.
Tell your doctor or pharmacistif you are taking this medicine. Your doctor may decide that you need extra monitoring.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Pregnancy
Apretude is not recommended during pregnancy. The effect of Apretude on pregnancy is unknown. Talk to your doctor: if you can be pregnant, if you plan to have a baby, or if you become pregnant. Do not stop attending appointments to receive Apretude without talking to your doctor. Your doctor will consider the benefit to you and the risk to your baby before starting/continuing Apretude.
Breastfeeding
It is unknown if the components of Apretude can pass into breast milk. However, it is possible that cabotegravir may pass into breast milk during the 12 months following the last Apretude injection. If you are breastfeeding or think you may breastfeed, talk to your doctor. Your doctor will consider the benefit and risk of breastfeeding for you and your baby.
Driving and using machines
Apretude may cause dizziness and have other side effects that may make you less alert.
Do not drive or use machinesunless you are sure it won’t affect you.
3. How Apretude is given
This medicine is given as a 600 mg injection. A nurse or doctor will give you Apretude in the muscle of your buttock.
You must have an HIV test and be negativebefore receiving Apretude.
You will be given your first and second doses of Apretude one month apart. After the second dose, you will be given a single injection of Apretude every 2 months.
Before starting treatment with Apretude injectable, you and your doctor may decide to start with oral cabotegravir tablets (called an oral lead-in period) first. The oral lead-in period allows you and your doctor to see if it is suitable to switch to injections.
If you decide to start with the tablets:
- You must take one 30 mg Apretude tablet once a day for about a month.
- You must receive your first injection on the same day you take your last tablet or within 3 days after.
- After that, you will receive an injection every 2 months.
Injection schedule for every 2 months
When | Which medicine |
First and second injection, one month apart | Apretude 600 mg |
Third injection onwards, every 2 months | Apretude 600 mg |
If you are given too much Apretude injectable
This medicine will be given to you by a doctor or nurse, so it is unlikely that you will be given too much. If you are concerned, talk to your doctor or nurse and you will receive the necessary treatment.
If you miss an appointment to receive an Apretude injection
Contact your doctor immediatelyto schedule a new appointment.
It is essential that you attend your scheduled appointments to receive your injection and reduce your risk of getting HIV (see section 2). Talk to your doctor if you are thinking of stopping Apretude.
Talk to your doctor if you think you will not be able to receive your Apretude injection as scheduled. Your doctor may recommend that you take cabotegravir tablets instead until you can receive your Apretude injection again.
Do not stop receiving Apretude injections without your doctor’s advice.
Keep receiving Apretude injections for as long as your doctor recommends. Do not stop unless your doctor tells you to. If you stop treatment and you are still at risk of getting HIV, your doctor should start you on another medicine for PrEP during the 2 months after your last Apretude injection.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Do not receive your next Apretude injection and seek medical attention immediatelyif you notice any of the following symptoms:
- red patches that are not raised, target-like or circular patches on the trunk, often with blisters in the center, skin peeling, sores in the mouth, throat, nose, genitals, and eyes. These severe skin reactions can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis). These severe skin reactions are very rare (they may affect up to 1 in 10,000people).
Allergic reactions
Apretude contains cabotegravir, which is an integrase inhibitor. Integrase inhibitors, including cabotegravir, can cause a severe allergic reaction known as a hypersensitivity reaction.
If you experience any of the following symptoms:
- skin reaction
- high temperature (fever)
- lack of energy (fatigue)
- swelling, sometimes of the face or mouth (angioedema), which can cause difficulty breathing
- muscle or joint pain.
See a doctor immediately.Your doctor may consider it necessary to do tests to check your liver, kidneys, or blood and may tell you to stop taking Apretude.
Very common side effects(may affect more than 1 in 10 people)
- headache
- diarrhea
- injection site reactions
- very common: pain (which can rarely include temporary difficulty walking) and discomfort, a lump or hardened area
- common: redness (erythema), itching (pruritus), swelling, warmth, numbness (anesthesia), or bruising (which can include discoloration or blood accumulation under the skin)
- uncommon: pus accumulation (abscess)
- feeling hot (pyrexia)
- changes in liver function (increased transaminases), measured in blood tests.
Common side effects(may affect up to 1 in 10 people)
- depression
- anxiety
- abnormal dreams
- difficulty sleeping (insomnia)
- dizziness
- feeling sick (nausea)
- vomiting
- stomach pain (abdominal pain)
- gas (flatulence)
- rash
- muscle pain (myalgia)
- lack of energy (fatigue)
- general discomfort.
Uncommon side effects(may affect up to 1 in 100 people)
- suicidal thoughts and attempts (especially in users with a history of depression or mental health problems)
- allergic reaction (hypersensitivity)
- hives (urticaria)
- swelling, sometimes of the face or mouth (angioedema), which can cause difficulty breathing
- feeling drowsy (somnolence)
- weight gain
- feeling dizzy during or after an injection (vasovagal reactions). This can lead to fainting
- liver damage (hepatotoxicity). The signs may include yellowing of the skin and the whites of the eyes, loss of appetite, itching, stomach tenderness, pale stools, or unusually dark urine
- increased bilirubin in the blood, a breakdown product of red blood cells, measured in blood tests.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Apretude
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
The doctor or nurse is responsible for storing this medicine correctly.
Do not freeze.
6. Container Content and Additional Information
Apretude Composition
- The active ingredient is cabotegravir
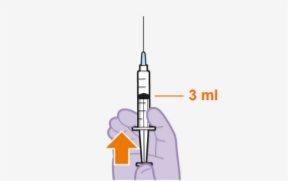
Each 3 ml vial contains 600 mg of cabotegravir.
The other components are:
Mannitol (E421)
Polysorbate 20 (E432)
Macrogol (E1521)
Water for injectable preparations
Product Appearance and Container Content
Cabotegravir is a white to light pink suspension, presented in a topaz-colored glass vial with a rubber stopper and an aluminum seal with a flip-off plastic cap.
Marketing Authorization Holder
ViiV Healthcare BV
Van Asch van Wijckstraat 55H,
3811 LP Amersfoort
Netherlands
Manufacturer
GlaxoSmithKline Manufacturing S.p.A.
Strada Provinciale Asolana 90
Torrile
PR
43056
Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien ViiV Healthcare srl/bv Tel: + 32 (0) 10 85 65 00 | Lithuania ViiV Healthcare BV Tel: + 370 80000334 |
Bulgaria ViiV Healthcare BV Tel: + 359 80018205 | Luxembourg/Luxemburg ViiV Healthcare srl/bv Belgium/Belgien Tel: + 32 (0) 10 85 65 00 |
Czech Republic GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Hungary ViiV Healthcare BV Tel: + 36 80088309 |
Denmark GlaxoSmithKline Pharma A/S Tel: + 45 36 35 91 00 | Malta ViiV Healthcare BV Tel: + 356 80065004 |
Germany ViiV Healthcare GmbH Tel: + 49 (0)89 203 0038-10 | Netherlands ViiV Healthcare BV Tel: + 31 (0) 33 2081199 |
Estonia ViiV Healthcare BV Tel: + 372 8002640 | Norway GlaxoSmithKline AS Tel: + 47 22 70 20 00 |
Greece GlaxoSmithKline Μονοπρóσωπη A.E.B.E. Tel: + 30 210 68 82 100 | Austria GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
Spain Laboratorios ViiV Healthcare, S.L. Tel: + 34 900 923 501 | Poland GSK Services Sp. z o.o. Tel: + 48 (0)22 576 9000 |
France ViiV Healthcare SAS Tel: + 33 (0)1 39 17 69 69 | Portugal VIIVHIV HEALTHCARE, UNIPESSOAL, LDA Tel: + 351 21 094 08 01 |
Croatia ViiV Healthcare BV Tel: + 385 800787089 | Romania ViiV Healthcare BV Tel: + 40 800672524 |
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenia ViiV Healthcare BV Tel: + 386 80688869 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovak Republic ViiV Healthcare BV Tel: + 421 800500589 |
Italy ViiV Healthcare S.r.l Tel: + 39 (0)45 7741600 | Finland GlaxoSmithKline Oy Tel: + 358 (0)10 30 30 30 |
Cyprus ViiV Healthcare BV Tel: + 357 80070017 | Sweden GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvia ViiV Healthcare BV Tel: + 371 80205045 | United Kingdom (Northern Ireland) ViiV Healthcare BV Tel: + 44 (0)800 221441 |
Date of Last Revision of this Leaflet:{MM/YYYY}.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
This information is intended only for healthcare professionals:
| |
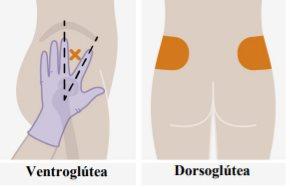
Overview One injection is required per visit; 3 ml of cabotegravir (600 mg). Cabotegravir is a suspension that does not require additional dilution or reconstitution. Cabotegravir is for intramuscular use only. It should be administered in the gluteal area. Note:The ventrogluteal area is recommended. | |
Storage Information | |
Do notfreeze. | |
To Prepare the Injection | |
| |
To Administer the Injection | |
Consider the patient's constitution and medical judgment to choose the appropriate needle length. | |
You Will Also Need | |
| |
Preparation | |
| |
|
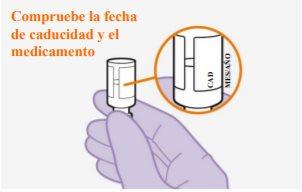
Do not useif the expiration date has passed
Note:The cabotegravir vial is made of topaz-colored glass. |
| |
|
|
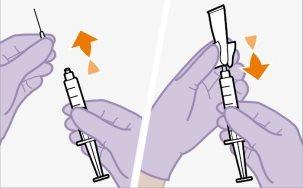
Do notallow anything to touch the rubber stopper after cleaning. | |
| |
|
|
| |
|
|
Note:Check that the suspension appears uniform and is white to light pink in color. | |
| |
|
|
Injection | |
| |
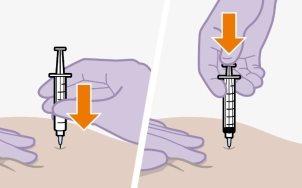
| Injections should be administered in the gluteal area. Choose between the following areas for injection:
|
Note:For intramuscular use in the gluteal area only. Do notadminister intravenously. | |
| |
|
|
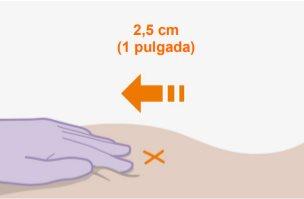
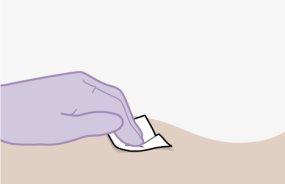
Note:Clean the injection site with an alcohol-impregnated swab. Allow the skin to air dry before proceeding. | |
| |
|
|
| |
|
|
| |
|
Do notmassage the area. |
Questions and Answers | |
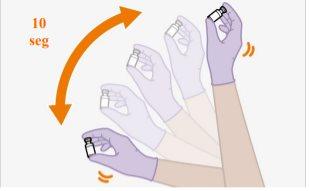
You should wait at least 15 minutes before administering the injection to allow the medication to reach room temperature. It is best to let the vial reach room temperature naturally. However, you can use the heat from your hands to speed up the warming time, but make sure the vial does not exceed 30°C. Do not use any other heating method.
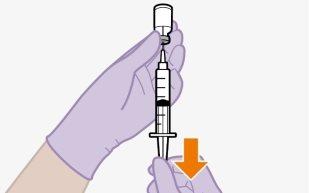
It is best to inject the medication (at room temperature) as soon as possible after withdrawal. However, the medication can remain in the syringe for up to 2 hours before injection. If the medication remains in the syringe for more than 2 hours, the filled syringe and needle must be discarded.
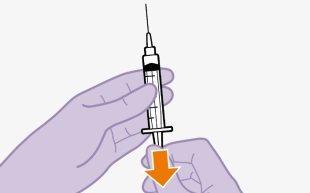
Injecting 1 ml of air into the vial facilitates the withdrawal of the dose with the syringe. Without the air, some of the liquid may return to the vial unintentionally, leaving less medication in the syringe than intended.
Administration in the ventrogluteal area is recommended because it is farther from major nerves and blood vessels. Administration in the dorsogluteal area, in the gluteus maximus muscle, is also acceptable if preferred by the healthcare professional. The injection should not be administered in any other area. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to APRETUDE 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: TABLET, 30 mgActive substance: cabotegravirManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 30 mgActive substance: cabotegravirManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: INJECTABLE, 600 mgActive substance: cabotegravirManufacturer: Viiv Healthcare B.V.Prescription required
Online doctors for APRETUDE 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about APRETUDE 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions