
AMICACIN BRAUN 500 mg/2 ml INJECTABLE SOLUTION

How to use AMICACIN BRAUN 500 mg/2 ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Amicacina Braun 500 mg/2ml Solution for Injection
Read all of this leaflet carefully before you start using this medicine
|
Contents of the pack:
- What Amicacina Braun 500 mg/2ml is and what it is used for
- What you need to know before you use Amicacina Braun 500 mg/2ml
- How to use Amicacina Braun 500 mg/2ml
- Possible side effects
- Storage of Amicacina Braun 500 mg/2ml
- Contents of the pack and other information
1. What Amicacina Braun 500 mg/2ml is and what it is used for
Amicacina Braun 500 mg/2ml is an aqueous solution of amikacin, a bactericidal antibiotic of the aminoglycoside group.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or the common cold.
It is essential to follow the instructions regarding dosage, administration, and duration of treatment as indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw away medications via wastewater or trash.
It is used for the short-term treatment of severe infections caused by sensitive microorganisms. It is primarily used in the following cases:
- Blood infection, also known as sepsis
- Severe lower respiratory tract infections
- Infections of the central nervous system (including meningitis)
- Intra-abdominal infections, including peritonitis
- Infections of the skin, bones, and soft tissues (including burns)
- Complicated and recurrent urinary tract infections
- Infections after surgery
2. What you need to know before you use Amicacina Braun 500 mg/2ml
Do not use Amicacina Braun 500 mg/2ml:
- If you are allergic to amikacin or other aminoglycoside antibiotics,
- If you have myasthenia gravis,
- It should not be administered with other products that are toxic to the ear or kidney, or with potent diuretics,
Warnings and precautions:
Consult your doctor, pharmacist, or nurse before starting treatment with Amicacina Braun 500 mg/2ml.
Inform your doctor of any allergies or medical problems you have or have had, especially:
- if you are allergic to amikacin or other aminoglycoside antibiotics,
- if your kidneys do not function properly, as this may increase the risk of toxicity,
- if you have muscle disorders, such as myasthenia gravis or Parkinson's disease, as this may worsen muscle weakness,
- if you are taking diuretics that may be toxic to the ear,
- if symptoms of ear toxicity such as dizziness, vertigo, tinnitus (ringing in the ears), buzzing in the ears, and hearing loss, or symptoms of kidney toxicity appear.
- if severe diarrhea appears,
- it is possible that your infection may not respond to amikacin if it did not respond to other aminoglycosides, and you may have an allergic reaction if you are already allergic to another aminoglycoside,
- if you or your family members have a mitochondrial mutation disease (a genetic disease) or hearing loss due to antibiotics, you are advised to inform your doctor or pharmacist before taking an aminoglycoside; certain mitochondrial mutations may increase your risk of hearing loss with this medication. Your doctor may recommend genetic testing before administration of Amicacina Braun 500 mg/2ml,
- amikacin may alter the values of some substances in analysis, such as urea nitrogen, transaminases, alkaline phosphatase, bilirubin, creatinine, lactate dehydrogenase, sodium, potassium, magnesium, and calcium.
To reduce the risk of nerve damage to the ear and kidney, your doctor will be especially careful in evaluating the following:
- Monitoring of hearing, balance, and kidney function before, during, and after treatment.
- Dosage strictly according to kidney function.
- If you have altered kidney function, the total dose of antibiotics administered additionally directly to the site of infection will be taken into account.
- Monitoring of amikacin serum concentrations during treatment if the details of your case require it.
- If you already have nerve damage in the ear (hearing or balance impairment), or if treatment is long-term, additional monitoring of balance and hearing function is required.
- In case possible, you will receive amikacin treatment for no more than 10-14 days (normally, 7-10 days).
- A sufficient amount of time, 7-14 days, should pass between individual treatments with amikacin and other closely related antibiotics.
- Avoid administering other substances with potential harmful effects on the ear or kidneys in combination with amikacin. If this is unavoidable, careful monitoring of kidney function is required.
- The level of body fluids and urine production should be within the normal range.
Children
This medication will be administered with caution and only if there is no other alternative in premature and newborn patients due to the incomplete renal development of these patients.
Other medications and Amicacina Braun 500 mg/2ml
Inform your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
Administration of Amicacina Braun 500 mg/2ml together with the following medications may require modifying the dose of one of them or interrupting treatment.
- Other aminoglycoside antibiotics (gentamicin, tobramycin...) or capreomycin.
- Amphotericin, vancomycin, agents that decrease immunity, cytotoxic agents (toxic to cells such as cyclosporine or cisplatin), cephalosporins (cephalothin), or potent diuretics.
- Halogenated hydrocarbon anesthetics by inhalation, massive blood transfusions, and neuromuscular blockers.
- Antihistamines, buclizine, cyclizine, loxapine, meclozine, phenothiazines, thioxanthenes, or trimethobenzamide.
- Anti-myasthenic agents (medications for treating myasthenia gravis - muscle weakness).
- Indomethacin.
- Malathion.
- Polypeptide antibiotics (colistin, polymyxin).
- Opioid analgesics.
- Beta-lactam antibiotics (penicillin).
- Intravenous indomethacin in children.
Pregnancy and breastfeeding:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Treatment during pregnancy is not recommended, although your doctor will assess the convenience of its use. If the medication is used during pregnancy, or if you become pregnant during treatment, you should be informed of the possible risks.
There is no data on excretion into breast milk, but as a general rule, breastfeeding should not be done if the mother is undergoing treatment.
Driving and using machines:
There is no evidence of effects on the ability to drive vehicles or use machinery. However, this ability may be altered if adverse reactions such as dizziness, vertigo, and lethargy appear.
Amicacina Braun 500 mg/2ml contains sodium metabisulfite (E-223), methylparaben (E-218), and propylparaben (E-216).
This medication may cause severe allergic reactions and bronchospasm (sudden feeling of suffocation) because it contains sodium metabisulfite (E-223).
It may cause allergic reactions (possibly delayed) and, exceptionally, bronchospasm (sudden feeling of suffocation) because it contains methylparaben (E-218) and propylparaben (E-216).
This medication contains less than 23 mg of sodium (1 mmol) per 2 ml vial; that is, it is essentially "sodium-free".
3. How to use Amicacina Braun 500 mg/2ml
Follow the instructions for administration of the medication contained in this leaflet or as indicated by your doctor, pharmacist, or nurse. If in doubt, ask your doctor, pharmacist, or nurse.
Your doctor will indicate the duration of your treatment. Do not stop treatment before completion.
Your doctor will determine the most suitable dose for you, according to your age, weight, general condition, severity of the infection, and kidney function. Kidney function should be monitored during treatment.
Maximum concentrations (30-90 minutes after injection) above 35 micrograms/min and minimum concentrations (just before the next dose) above 10 micrograms/min should be avoided.
If kidney function is normal, the recommended dose for adults is 15-20 mg/kg/day divided into a single daily dose or divided into 2 or 3 equal doses administered at equivalent intervals.
Use in children
Adolescents (12 to less than 18 years) and children (2 to 11 years):
The recommended dosage is the same as for adults.
Full-term newborns 2 weeks or older and infants (28 days to 23 months):
The recommended dose in children over 2 weeks is 7.5 mg/kg every 12 hours or 5 mg/kg every 8 hours.
Full-term newborns less than 2 weeks (0 to 13 days):
A loading dose of 10 mg/kg will be administered, followed by 7.5 mg/kg every 12 hours.
Preterm infants:
The recommended dose is 7.5 mg/kg every 12 hours.
Amicacina Braun 500 mg/2ml may not be suitable for adjusting the dosage in all cases. There are other presentations of solution for intravenous infusion that can be used in this situation.
If the patient has altered kidney function, the doctor will carefully monitor it and, if necessary, modify the dose or extend the intervals between doses.
Amikacina Braun 500 mg/2 ml should be administered intramuscularly.
If you use more Amicacina Braun 500 mg/2ml than you should:
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or contact the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested. It is recommended to bring the packaging and the leaflet of the medication to the healthcare professional.
In case of a toxic reaction due to high dosing or accumulation, especially in patients with severe kidney failure, peritoneal dialysis or hemodialysis may facilitate the elimination of the antibiotic.
If a hypersensitivity reaction occurs, its administration will be discontinued, and the patient will receive specific treatment according to the nature and intensity of the reaction (antihistamines, corticosteroids, adrenaline...)
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Amicacina Braun 500 mg/2ml can cause side effects, although not everybody gets them.
The following is a list of adverse reactions by system organ class and frequency, according to the following criteria:
Very common (> 1/10), common (>1/100, < 1/10), uncommon (>1/1000, <1/100), rare (>1/10000, < 1/1000), and very rare (<1/10000)
Renal and urinary disorders: | very common: renal toxicity: increased urea nitrogen and non-protein nitrogen and creatinine in blood, albuminuria, presence of red and white blood cells in urine… |
Ear and labyrinth disorders: | very common: toxicity of the nervous system and ear: hearing loss, vertigo, cochlear damage including high-frequency hearing loss. Dizziness, ataxia (disease affecting voluntary movements), vertigo, tinnitus (ringing in the ears), and hearing loss may occur. |
Nervous system disorders: | very common: toxicity of the nervous system and neuromuscular blockade: acute muscle paralysis and apnea (suspension of breathing), numbness, tingling, muscle spasms, and convulsions. uncommon: headache, tremors. |
Skin and subcutaneous tissue disorders: | uncommon: skin rash, redness, and elevated temperature at the injection site. |
Gastrointestinal disorders: | uncommon: nausea, vomiting. |
Musculoskeletal and connective tissue disorders: | uncommon: paresthesia (sensation of numbness, tingling, or burning in the skin), arthralgia (joint pain). |
General disorders and administration site conditions: | uncommon: pain at the injection site. |
Blood and lymphatic system disorders: | rare: eosinophilia, anemia (low red blood cell concentration). |
Cardiac disorders: | rare: hypotension (low blood pressure); hypomagnesemia (low magnesium level). |
Reporting of suspected adverse reactions
It is important to report any suspected adverse reactions to the medication after authorization. This allows for continuous monitoring of the benefit/risk ratio of the medication. Healthcare professionals are invited to report any suspected adverse reactions via the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es
5. Storage of Amicacina Braun 500 mg/2ml
Keep out of sight and reach of children.
Store in a cool place and in the original packaging to protect it from light.
Do not use Amicacina Braun 500 mg/2ml after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
The contents of the vials should be used immediately after opening. Once the package is opened, discard the unused portion of the solution.
Medicines should not be disposed of via wastewater or trash. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Container Content and Additional Information
Composition ofAmicacina Braun 500 mg/2ml
The active ingredient of Amicacina Braun 500 mg/2ml is amikacin sulfate
Each vial contains 500 mg of amikacin base.
The other components are: methylparaben (E-218), propylparaben (E-216), sodium metabisulfite (E-223), disodium edetate, and water for injectable preparations.
Appearance of the Product and Container Content
It is presented in containers containing 1 and 50 glass vials of 2 ml.
Marketing Authorization Holder and Manufacturer
- Braun Medical, S.A.
Ctra. de Terrassa, 121
08191-Rubí (Barcelona)
Spain
Date of the Last Revision of this Prospectus:June 2023
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
----------------------------------------------------------------------------------------------
This information is intended solely for doctors or healthcare professionals:
Renal and auditory nerve functions should be carefully monitored in patients with renal insufficiency when used for long periods or when administered in doses higher than recommended, as disorders of the VIII cranial nerve and renal function have been reported. The appearance of signs of nephrotoxicity or ototoxicity will determine a readjustment of the dosage or the suspension of treatment according to the cases.
Amikacin plasma levels should be studied, adjusting the dosage to avoid prolonged levels above 35 micrograms/ml. Urine should be examined to detect increases in protein excretion, the presence of cells or cylinders, and a decrease in density.
Ototoxicity in children is not well determined.
In the event of superinfections produced by resistant microorganisms, treatment should be suspended and adequate therapy applied.
Patients should be well-hydrated during treatment.
Dosage
Patients with normal renal function can receive a single daily dose, as long as the maximum concentration does not exceed 35 mg/ml. The usual duration of treatment is 7 to 10 days.
In difficult and complicated infections, a longer treatment may be necessary. In these cases, renal, auditory (cochlear), and vestibular functions will be monitored.
The recommended dose for adults, adolescents, and children over 2 years is 15-20 mg/kg/day in the form of a single daily dose or divided into two or three equal doses administered at equivalent intervals (i.e., for a dose of 15 mg/kg/day, it is administered divided into doses of 7.5 mg/kg every 12 hours or 5 mg/kg every 8 hours).
The total dose should not exceed 1.5 g/day.
Pediatric Population
Full-term newborns 2 weeks or older and infants (28 days to 23 months):
The recommended dose in children over 2 weeks is 7.5 mg/kg every 12 hours or 5 mg/kg every 8 hours.
Full-term newborns under 2 weeks (0 to 13 days):
A first loading dose of 10 mg/kg will be administered, followed by 7.5 mg/kg every 12 hours.
Preterm infants:
The recommended dose is 7.5 mg/kg every 12 hours.
Amicacina Braun 500 mg/2ml may not be suitable for adjusting the dosage in all cases. There are other presentations of solution for intravenous infusion that can be used in this situation.
Special Populations
Renal Insufficiency
In patients with renal insufficiency, measured by a creatinine clearance of <50 ml min, it is not recommended to administer amikacin in a single dose, as these patients would have prolonged exposure high trough concentrations.< p>
The dosage may be adjusted by either increasing the interval between doses or administering reduced doses.
Dosage adjustment based on serum creatinine values:
- Increasing the dosing interval, to the normal dose:
The increase in the interval in hours for a normal dosing (7.5 mg/kg every 12 hours) can be calculated by multiplying the serum creatinine value by 9. For example, if the serum creatinine value is 2 mg/100 ml, the recommended dosage would be 7.5 mg/kg every 18 hours.
- Modifying the dose, maintaining the periodicity between administrations:
Initially, the normal dose of 7.5 mg/kg will be administered as a loading dose. To determine the maintenance dose to be administered every 12 hours, the dose should be reduced in proportion to the reduction in creatinine clearance:
Maintenance dose (every 12 hours):
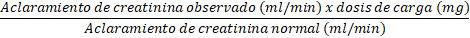
Patients with Obesity
The dose should be adjusted according to body weight and renal function. In obese patients, the initial dose should be calculated with the ideal weight plus 40% excess weight.
Patients with Burns and Severe Infections
They may require a higher dose administration or at intervals of four to six hours, since in these cases the half-life of the drug is shorter.
Method of Administration
Direct intramuscular route.
Monitoring Recommendations:
For the calculation of the correct dose, the following should be taken into account:
- The patient's weight before treatment.
- The state of renal function by determining the serum creatinine concentration or creatinine clearance. Renal function should be monitored during treatment.
Whenever possible, amikacin serum concentrations should be determined to ensure adequate levels. It is recommended to measure intermittent minimum and maximum serum concentrations during treatment. Maximum concentrations (30-90 minutes after injection) above 35 µg/ml and minimum concentrations (just before the next dose) above 10 µg/ml should be avoided.
Aminoglycosides should be administered separately, regardless of their route of administration, and should not be physically pre-mixed with other medications.
MEDICATION SUBJECT TO MEDICAL PRESCRIPTION
HOSPITAL USE
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMICACIN BRAUN 500 mg/2 ml INJECTABLE SOLUTIONDosage form: INJECTABLE PERFUSION, 1000 mgActive substance: amikacinManufacturer: B Braun Medical S.A.Prescription requiredDosage form: INJECTABLE INFUSION, 500 mgActive substance: amikacinManufacturer: B Braun Medical S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 5 mg/mlActive substance: amikacinManufacturer: Fresenius Kabi España, S.A.U.Prescription required
Online doctors for AMICACIN BRAUN 500 mg/2 ml INJECTABLE SOLUTION
Discuss questions about AMICACIN BRAUN 500 mg/2 ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















