ALTUVOCT 750 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use ALTUVOCT 750 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ALTUVOCT 250 UI powder and solvent for solution for injection
ALTUVOCT 500 UI powder and solvent for solution for injection
ALTUVOCT 750 UI powder and solvent for solution for injection
ALTUVOCT 1,000 UI powder and solvent for solution for injection
ALTUVOCT 2,000 UI powder and solvent for solution for injection
ALTUVOCT 3,000 UI powder and solvent for solution for injection
ALTUVOCT 4,000 UI powder and solvent for solution for injection
efanesoctocog alfa (recombinant human coagulation factor VIII)
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is ALTUVOCT and what is it used for
- What you need to know before you use ALTUVOCT
- How to use ALTUVOCT
- Possible side effects
- Storage of ALTUVOCT
- Contents of the pack and further information
1. What is ALTUVOCT and what is it used for
ALTUVOCT contains the active substance efanesoctocog alfa, a factor VIII replacement protein.
ALTUVOCT is used to treat and prevent bleeding episodes in patients with haemophilia A (a bleeding disorder caused by a lack of factor VIII) and can be used in patients of all ages.
Factor VIII is a protein that is naturally present in the body and is necessary for blood to clot and stop bleeding. In patients with haemophilia A, factor VIII is missing or does not work properly.
ALTUVOCT replaces this missing or defective factor VIII. ALTUVOCT increases the factor VIII levels in the blood, helping the blood to clot at the site of bleeding, which temporarily corrects the tendency to bleed.
2. What you need to know before you use ALTUVOCT
Do not use ALTUVOCT
- if you are allergic to efanesoctocog alfa or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting treatment with ALTUVOCT.
- There is a rare possibility that you may experience an anaphylactic reaction (a severe and sudden allergic reaction) to ALTUVOCT. Signs of allergic reactions include generalised itching, hives, feeling of chest tightness, difficulty breathing, and low blood pressure. If any of these symptoms occur, stop the injection immediately and contact your doctor.
- Talk to your doctor if you think you are not getting control of your bleeding or your child's bleeding with the dose you are getting, as there may be several reasons for this. In some people using this medicine, antibodies against factor VIII (also known as factor VIII inhibitors) may develop. The formation of factor VIII inhibitors is a known complication that can occur during treatment with all factor VIII medicines. These inhibitors, especially at high levels, can prevent the treatment from working properly; you or your child will be closely monitored for the development of these inhibitors.
Cardiovascular events
If you have heart disease or are at risk of heart disease, be careful when using factor VIII medicines and talk to your doctor.
Catheter-related complications
If you need a central venous access device (CVAD), consider the risk of CVAD-related complications, including local infections, bacteria in the blood, and thrombosis at the catheter insertion site.
Other medicines and ALTUVOCT
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, talk to your doctor before using this medicine.
Driving and using machines
ALTUVOCT has no or negligible influence on the ability to drive and use machines.
3. How to use ALTUVOCT
Treatment with ALTUVOCT will be started by a doctor experienced in the care of patients with haemophilia A. ALTUVOCT is given by injection into a vein.
After receiving the necessary training in the correct injection technique, patients or caregivers can administer ALTUVOCT at home. Your doctor will calculate your dose (in international units [IU]). This will depend on your weight and whether it is used for prevention or treatment of bleeding.
Follow your doctor's instructions for administering this medicine exactly. If you are unsure, talk to your doctor, pharmacist, or nurse.
Keep a record
Each time you use ALTUVOCT, write down the date, the name of the medicine, and the batch number.
Prevention of bleeding
The usual dose of ALTUVOCT is 50 international units (IU) per kilogram of body weight. The injection is given once a week.
Treatment of bleeding
The dose of ALTUVOCT is 50 international units (IU) per kilogram of body weight. The dose and frequency may be adjusted based on the severity and location of the bleeding.
Use in children and adolescents
ALTUVOCT can be used in children of all ages; the recommended dose is the same as for adults.
How to administer ALTUVOCT
ALTUVOCT is given by injection into a vein. See "Instructions on how to use ALTUVOCT" for more information.
If you use more ALTUVOCT than you should
Tell your doctor as soon as possible. Follow your doctor's instructions for administering ALTUVOCT exactly. If you are unsure, talk to your doctor, pharmacist, or nurse.
If you forget to use ALTUVOCT
Do not inject a double dose to make up for forgotten doses. Inject your dose as soon as you remember and then continue with your normal dosing schedule. If you are unsure, talk to your doctor, pharmacist, or nurse.
If you stop treatment with ALTUVOCT
If you stop treatment with ALTUVOCT, you may no longer be protected against bleeding or an existing bleed may not stop. Do not stop treatment with ALTUVOCT without talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If an anaphylactic reaction occurs, the injection should be stopped immediately and your doctor should be contacted immediately.
Signs of anaphylactic reactions include:
|
|
Risk of inhibitor formation
In children who have not received prior treatment with factor VIII medicines, the formation of inhibitory antibodies (see section 2) is very common (may affect more than 1 in 10 patients); however, in patients who have received prior treatment with factor VIII (more than 150 days of treatment), the risk is uncommon (may affect up to 1 in 100 patients). If you or your child develop inhibitory antibodies, the medicine may stop working properly and you or your child may experience persistent bleeding. If this happens, contact your doctor immediately.
The following side effects may occur with this medicine.
Very common side effects (may affect more than 1 in 10 people)
- headache
- arthralgia (joint pain)
Common side effects (may affect up to 1 in 10 people)
- pain in the limbs (arms, hands, legs, or feet)
- back pain
- eczema (itching, redness, or dryness of the skin)
- rash
- urticaria (itchy rash)
- fever
- vomiting
Uncommon side effects (may affect up to 1 in 100 people)
- reactions at the injection site (including bruising and inflammation)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ALTUVOCT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial after "EXP". The expiry date is the last day of the month stated.
Store in a refrigerator (2°C to 8°C).
Do not freeze.
Keep the vial in the outer carton in order to protect from light.
Before reconstitution, the ALTUVOCT powder can be stored at room temperature (≤ 30°C) for a single period not exceeding 6 months. The date of removal from the refrigerator should be written on the carton. After storage at room temperature, the medicine should not be returned to the refrigerator.
The medicine should not be used after the expiry date printed on the vial or 6 months after removal from the refrigerator, whichever is the earlier.
Once the ALTUVOCT powder has been dissolved in the solvent provided in the pre-filled syringe, it should be used immediately. Do not refrigerate the prepared solution.
After reconstitution, the solution should be clear and colourless to slightly opalescent. Do not use this medicine if you notice it is cloudy or contains visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
ALTUVOCT Composition
- The active ingredient is efanesoctocog alfa (recombinant human coagulation factor VIII).
Each ALTUVOCT vial nominally contains 250, 500, 750, 1,000, 2,000, 3,000, or 4,000 IU of efanesoctocog alfa.
- The other components are sucrose, calcium chloride dihydrate, histidine, arginine hydrochloride, and polysorbate 80.
Product Appearance and Container Contents
ALTUVOCT is presented as a powder and solvent for solution for injection. The powder is a loose or compact powder that is white to off-white in color. The solvent supplied for preparation of the solution for injection is a clear and colorless solution. After preparation, the solution for injection is clear and colorless to slightly opalescent.
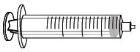
Each ALTUVOCT container contains 1 vial of powder, 3 mL of solvent in a prefilled syringe, 1 plunger rod, 1 vial adapter, and 1 infusion set.
Marketing Authorization Holder and Manufacturer
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm
Sweden
Swedish Orphan Biovitrum AB (publ)
Norra Stationsgatan 93
113 64 Stockholm
Sweden
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
Turn the leaflet over to see instructions for preparation and administration of the medicinal product.
Instructions on How to Use ALTUVOCT
READ THESE INSTRUCTIONS CAREFULLY BEFORE USING ALTUVOCT
ALTUVOCT is administered by intravenous injection after dissolving the injectable powder with the solvent supplied in the prefilled syringe.
If your dose requires more than one vial, you will receive several containers and, ideally, a large syringe.
Your healthcare professional should teach you how to prepare and inject ALTUVOCT correctly before you use it for the first time. Ask your healthcare professional if you have any questions.
Important Information
Check that the name and dose of the medicinal product are correct and that you know the frequency of administration of ALTUVOCT.
Do not use the medicinal product if it has expired, been opened, or appears to be damaged.
ALTUVOCT must not be mixed with other injectable solutions.
ALTUVOCT should ideally be stored in the refrigerator. Allow the vial and solvent syringe to reach room temperature before use. Do not use external heat.
Check that none of the components are damaged before use; do not use them if they appear to be damaged.
All components are for single use only.
Wash your hands and clean a flat surface before preparing the kit. Place the syringe securely on a flat surface when not handling it.
Guide to Components (Included in the Box)
ALTUVOCT is reconstituted by dissolving the injectable powder (A) in the solvent supplied in the prefilled syringe (B). The ALTUVOCT solution is then administered using the infusion set (E).
|
|
|
|
|
|
(prefilled with solvent) |
|
|
|
Additional Components (Not Included in the Box)
Make sure you have alcohol swabs (F).
Your pharmacist may have provided a large syringe (G) separately to withdraw the solution from multiple vials into a single syringe. If you have NOT been provided with a large syringe, follow steps 6 to 8 to administer the solution from each syringe.
|
|
|
|
Reconstitution
| |
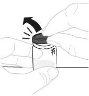
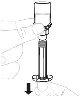
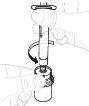
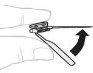
Hold the powder vial (A) over a clean and flat surface and remove the plastic cap. |
|
Clean the top of the vial with an alcohol swab. Make sure nothing comes into contact with the top of the vial once it has been cleaned. |
|
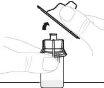
Remove the paper cover from the vial adapter package (D). Do not touch the vial adapter or remove it from its package. |
|
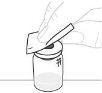
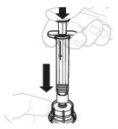
Place the vial adapter package directly over the top of the vial. Press firmly downward until the adapter is in place. The spike will puncture the vial stopper. |
|
| |
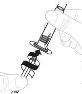
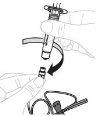
Insert the plunger rod (C) into the 3 mL syringe (B). Turn the plunger rod clockwise until it is securely attached. |
|
Separate the top part of the syringe cap from the 3 mL syringe by the perforations and set it aside. Do not touch the inside of the syringe cap or the tip of the syringe. |
|
| |
Lift the package to separate it from the vial adapter and discard it. |
|
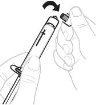
Hold the vial adapter by the lower end. Place the tip of the syringe over the top of the vial adapter. Turn the syringe clockwise until it is securely attached. |
|
| |
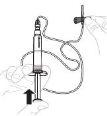
Slowly press the plunger rod to inject all of the solvent into the vial. |
|
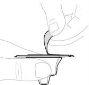
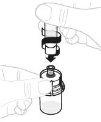
Gently swirl the vial in a circular motion with your thumb over the plunger rod until the powder is dissolved. Do not shake. |
|
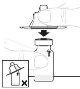
Inspect the solution before administration. It should be clear and colorless. Do not use the solution if it is cloudy or contains visible particles. | |
| |
If your dose requires more than one vial, follow the steps below (5a and 5b); otherwise, proceed to step 6. | |
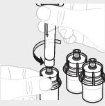
Repeat steps 1 to 4 with all vials until you have prepared enough solution for your dose. Remove the 3 mL syringes from each vial (see step 6b), leaving the solution in each vial. |
|
For each vial, attach the large syringe (G) to the vial adapter (see step 3b) and perform step 6 to combine the solution from each vial into the large syringe. If you only need part of a whole vial, use the graduated scale on the syringe to see the amount of solution you are withdrawing, as directed by your healthcare professional. |
|
| |
Hold the syringe upright. Slowly pull back the plunger rod to transfer all of the solution into the syringe. |
|
Detach the syringe from the vial by holding the vial adapter. Turn the syringe counterclockwise to detach it. |
|
Administration
| |
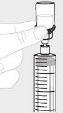
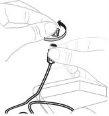
Open the infusion set package (E) (do not use if it is damaged). Remove the tube cap. Do not touch the exposed end of the tube. |
|
Attach the prepared syringe to the end of the infusion set tube by turning the syringe clockwise. |
|
If necessary, apply a tourniquet. Clean the injection site with an alcohol swab (F). |
|
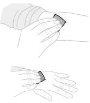
Remove air by holding the syringe upright and gently pressing the plunger rod. Do not push the solution through the needle. Air injection into a vein can be hazardous. |
|
| |
Remove the needle protective cover. Insert the needle into a vein, as directed by your doctor or nurse, and remove the tourniquet if applied. You can use a tourniquet to hold the plastic wings of the needle in place at the injection site to prevent it from moving. | |
The prepared solution should be injected intravenously over 1 to 10 minutes, depending on the patient's comfort level. | |
| |
Remove the needle. Fold the needle protector; it should click into place. |
|
Dispose of the used needle, any unused solution, the syringe, and the empty vial safely in an appropriate medical waste container. Do not reuse the equipment. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALTUVOCT 750 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for ALTUVOCT 750 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about ALTUVOCT 750 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions