
AKEEGA 100 MG/500 MG FILM-COATED TABLETS

How to use AKEEGA 100 MG/500 MG FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
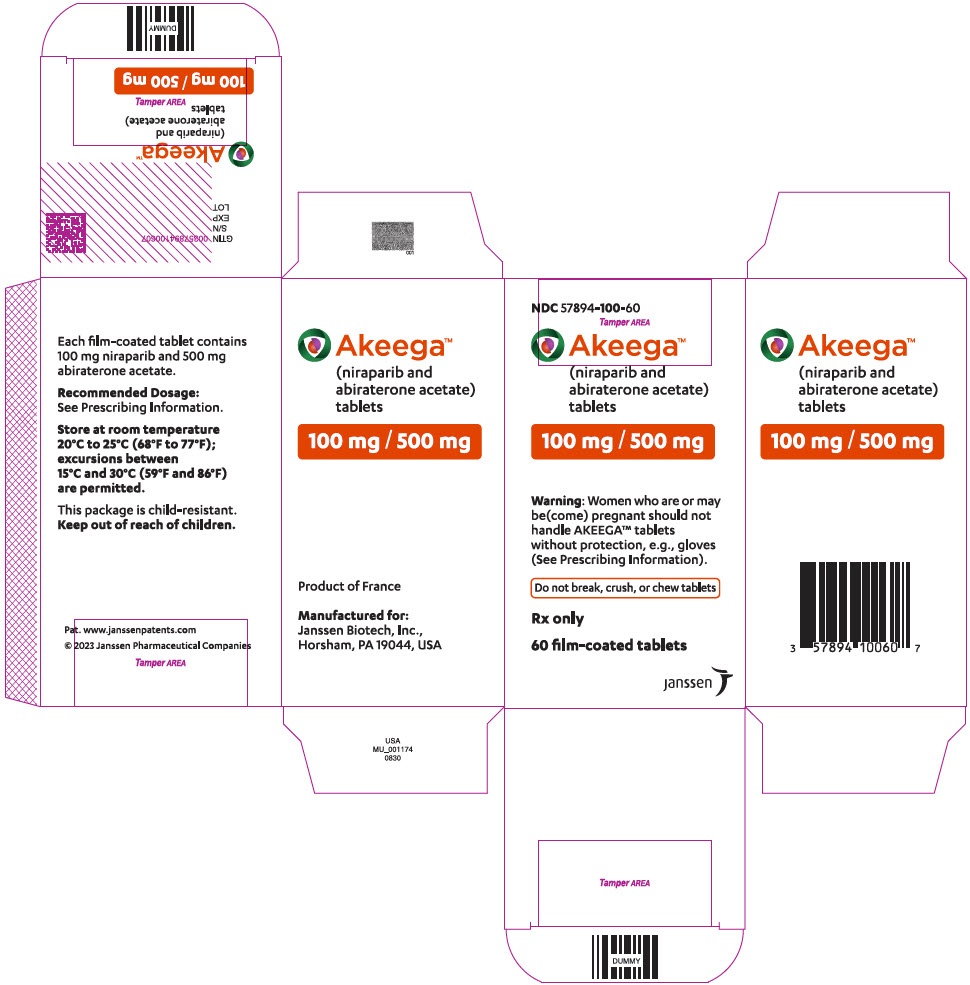
Akeega 100 mg/500 mg film-coated tablets
niraparib/abiraterone acetate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4).
Contents of the pack
- What is Akeega and what is it used for
- What you need to know before you take Akeega
- How to take Akeega
- Possible side effects
- Storage of Akeega
- Contents of the pack and other information
1. What is Akeega and what is it used for
Akeega is a medicine that contains two active substances: niraparib and abiraterone acetate and works in two different ways.
Akeega is used to treat adult men with prostate cancer that has spread to other parts of the body and is no longer responding to medical or surgical treatment that lowers testosterone (also called castration-resistant metastatic prostate cancer).
Niraparib is a type of cancer medicine called a PARP inhibitor. PARP inhibitors block an enzyme called poly(adenosine diphosphate-ribose) polymerase (PARP). PARP helps cells repair damaged DNA. When PARP is blocked, cancer cells cannot repair their DNA, which leads to the death of tumor cells, helping to control the cancer.
Abiraterone stops the body from making testosterone; this can slow down the growth of prostate cancer.
When taking this medicine, your doctor will also prescribe another medicine called prednisone or prednisolone, to reduce the chance of you getting high blood pressure, accumulating too much water in your body (fluid retention), or having low levels of a chemical called potassium in your blood.
2. What you need to know before you take Akeega
Do not take Akeega:
- if you are allergic to niraparib or abiraterone acetate or any of the other ingredients of this medicine (listed in section 6).
- if you are a woman who is pregnant or could become pregnant.
- if you have severe liver disease.
- in combination with treatment with Ra-223 (used for the treatment of prostate cancer). This is due to a possible increased risk of bone fracture or death.
Do not take this medicine if any of the above applies to you. If you are not sure, consult your doctor or pharmacist before taking this medicine.
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine or while taking this medicine if you have:
- a low blood cell count. Signs and symptoms to look out for include fatigue, fever, or infection, and unusual bruising or bleeding. Akeega can also lower your blood cell count. Your doctor will regularly perform blood tests during your treatment.
- high blood pressure or heart failure or low levels of potassium in the blood (low levels of potassium in the blood can increase the risk of heart rhythm problems), if you have had other heart or blood vessel problems, if you have a fast or irregular heartbeat, if you have difficulty breathing, if you have gained weight quickly or have swelling in your feet, ankles, or legs. Your doctor will regularly check your blood pressure during treatment.
- headaches, changes in vision, confusion, or seizures. These can be signs of a rare neurological side effect called posterior reversible encephalopathy syndrome (PRES) that has been associated with the use of niraparib, an active ingredient of Akeega.
- high fever, fatigue, and other signs and symptoms of severe infection.
- blood clots in the lungs, or if you have had them in the past.
- liver problems.
- low or high blood sugar levels.
- muscle weakness and/or muscle pain.
If any of the above applies to you (or you are not sure), consult your doctor or pharmacist before taking this medicine.
If you have a low blood cell count for a long time while taking Akeega, this can be a sign of more serious problems with the bone marrow, such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Your doctor may want to examine your bone marrow to detect these problems.
Before starting to take Akeega, also consult your doctor or pharmacist about:
- the effect Akeega can have on your bones.
- taking prednisone or prednisolone (another medicine you need to take with Akeega).
If you are not sure if any of the above applies to you, consult your doctor or pharmacist before taking this medicine.
Blood tests
Akeega may affect your liver, but you may not notice any symptoms of liver problems. Therefore, while you are taking this medicine, your doctor will regularly perform blood tests to check for any effect on your liver.
Children and adolescents
This medicine must not be used in children or adolescents. If a child or adolescent accidentally swallows Akeega, take them to the hospital immediately and bring this leaflet with you to show to the emergency doctor.
Other medicines and Akeega
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This is because Akeega can affect the way other medicines work. Also, other medicines can affect the way Akeega works.
Treatment with medicines that stop the body from making testosterone may increase the risk of heart rhythm problems. Tell your doctor if you are being treated with medicines:
- for heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol);
- that increase the risk of heart rhythm problems (e.g., methadone), used for pain relief and as part of drug addiction detoxification; moxifloxacin (an antibiotic); antipsychotics used for severe mental illnesses).
Consult your doctor if you are taking any of the medicines listed above.
Taking Akeega with food
- This medicine must not be taken with food (see section 3, "How to take Akeega"), as this may increase the risk of side effects.
Pregnancy and breastfeeding
Akeega is not indicated in women.
- This medicine may harm the fetus if taken by pregnant women.
- Pregnant women or women who may become pregnant should wear gloves if they need to touch or handle Akeega.
Contraception for men using Akeega
- If you have sex with a woman of childbearing age, you must use a condom and another effective method of contraception. Use contraceptive methods during treatment and for 4 months after stopping treatment. Consult your doctor if you have any questions about contraception.
- If you have sex with a pregnant woman, you must use a condom to protect the fetus.
Driving and using machines
Taking Akeega can make you feel weak, dizzy, tired, or faint. This could affect your ability to drive and use machines. Be careful when driving or using machines.
Akeega contains lactose and sodium
- Akeega contains lactose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
- This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How to take Akeega
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, consult your doctor or pharmacist again.
How much to take
The recommended starting dose is 200 mg/1,000 mg (two tablets) once a day.
How to take Akeega
- Take this medicine by mouth
- Do not take Akeega with food.
- Take the Akeega tablets as a single dose once a day on an empty stomach at least 1 hour before or at least 2 hours after eating (see section 2, "Taking Akeega with food").
- Swallow the tablets whole with water. Do not break, crush, or chew the tablets. This will ensure that the medicine works as well as possible.
- Akeega is given with another medicine called prednisone or prednisolone.
- Take prednisone or prednisolone exactly as your doctor tells you.
- You will need to take prednisone or prednisolone every day while you are taking Akeega.
- If you have a medical emergency, you may need to adjust the amount of prednisone or prednisolone you take. Your doctor will tell you if you need to change the amount of prednisone or prednisolone you take. Do not stop taking prednisone or prednisolone unless your doctor tells you to.
Your doctor may also prescribe other medicines while you are taking Akeega.
If you take more Akeega than you should
If you take more tablets than you should, contact your doctor. You may have a higher risk of side effects.
If you forget to take Akeega
If you forget to take Akeega or prednisone or prednisolone, take your normal dose as soon as you remember on the same day.
If you forget to take Akeega or prednisone or prednisolone for more than one day, consult your doctor immediately.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
If you stop taking Akeega
Do not stop taking Akeega or prednisone or prednisolone unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Stop taking Akeega and seek medical help immediately if you get any of the following symptoms:
Very common(may affect more than 1 in 10 people):
- Bleeding or bruising for longer than usual: can be signs of low platelet count (thrombocytopenia).
- Difficulty breathing, feeling very tired, pale skin, or fast heartbeat: can be signs of low red blood cell count (anemia).
- Fever or infection: a low white blood cell count (neutropenia) can increase the risk of infection. Signs can include fever, chills, feeling weak or confused, cough, pain, or burning when passing urine. Some infections can be severe and life-threatening.
- Muscle weakness, muscle contractions, or strong heartbeats (palpitations). These can be signs that the level of potassium in the blood is low (hypokalemia).
- Increased levels of the enzyme "alkaline phosphatase" in the blood.
Frequency not known(cannot be estimated from the available data): not reported with the use of Akeega but reported with the use of niraparib or abiraterone acetate (components of Akeega)
- Allergic reaction (including severe allergic reaction that can be life-threatening). Signs include: raised, itchy rash (hives) and swelling, sometimes of the face or mouth (angioedema), causing difficulty breathing and collapse or loss of consciousness.
- A sudden increase in blood pressure, which can be a medical emergency that can cause organ damage or can be life-threatening.
Other side effects
If you experience side effects, consult your doctor. These include:
Very common(may affect more than 1 in 10 people):
- urinary tract infection
- low white blood cell count (leucopenia), seen in blood tests
- decreased appetite
- difficulty sleeping (insomnia)
- feeling dizzy
- difficulty breathing
- constipation
- feeling sick (nausea)
- vomiting
- back pain
- joint pain
- feeling very tired
- feeling weak
- weight loss
- bone fractures
Common(may affect up to 1 in 10 people):
- pneumonia
- lung infection (bronchitis)
- infection of the nose and throat (nasopharyngitis)
- low count of a type of white blood cell (lymphopenia), seen in blood tests
- high levels of a type of fat (hypertriglyceridemia) in the blood
- depression
- feeling anxious
- headache
- fast heartbeat
- fast or irregular heartbeats (palpitations)
- irregular heartbeats (atrial fibrillation)
- heart failure, which causes difficulty breathing and swelling of the legs
- heart attack
- cough
- blood clot in the lungs, which causes chest pain and difficulty breathing
- inflammation in the lungs
- stomach pain
- indigestion
- diarrhea
- swelling
- mouth sores
- dry mouth
- inflammation in the liver (hepatitis), seen in blood tests
- rash
- muscle pain
- blood in the urine
- swelling of hands, ankles, or feet
- increased levels of "creatinine" in the blood
- increased levels of the enzyme "aspartate aminotransferase" in the blood
- increased levels of the enzyme "alanine aminotransferase" in the blood
Uncommon(may affect up to 1 in 100 people):
- severe infection (sepsis) that spreads from the urinary tract to the whole body
- inflammation in the eye (conjunctivitis)
- confusion
- difficulty thinking, remembering information, or solving problems (cognitive impairment)
- change in taste
- chest discomfort, often caused by physical activity
- abnormal electrocardiogram (ECG), which can be a sign of heart problems
- nosebleeds
- inflammation of the protective linings of body cavities, such as the nose, mouth, or digestive system
- sudden liver failure
- increased sensitivity of the skin to sunlight
- increased levels of "gamma-glutamyltransferase" in the blood
Frequency not known(cannot be estimated from the available data) - not reported with the use of Akeega but reported with the use of niraparib or abiraterone acetate (components of Akeega)
- low count of all types of blood cells (pancytopenia)
- brain disease with symptoms such as seizures (fits), headache, confusion, and changes in vision (posterior reversible encephalopathy syndrome or PRES), which is a medical emergency that can cause organ damage or can be life-threatening
- problems with the adrenal glands (related to salt and water problems) in which very little hormone is produced, which can cause problems such as weakness, tiredness, loss of appetite, nausea, dehydration, and skin changes
- inflammation in the lungs due to an allergic reaction (allergic alveolitis)
- muscle disease (myopathy), which can cause weakness, stiffness, or muscle spasms
- breakdown of muscle tissue (rhabdomyolysis), which can cause muscle pain or cramps, tiredness, and dark urine
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Akeega
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton (blister, inner carton, outer carton, and package) after EXP. The expiry date refers to the last day of the month shown.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Akeega Composition
- The active ingredients are niraparib and abiraterone acetate. Each film-coated tablet contains 100 mg of niraparib and 500 mg of abiraterone acetate.
- The other components of the tablet core are anhydrous colloidal silica, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The film coating contains red iron oxide (E172), yellow iron oxide (E172), sodium lauryl sulfate, glycerol monocaprylate, polyvinyl alcohol, talc, and titanium dioxide (E171) (see section 2, Akeega contains lactose and sodium).
Product Appearance and Container Content
The film-coated tablets of Akeega are orange, oval in shape, with
"N 100 A" engraved on one side and smooth on the other.
Each 28-day box contains 56 film-coated tablets in two cardboard boxes of 28 film-coated tablets each.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Cilag SpA
Via C. Janssen,
Borgo San Michele
Latina 04100
Italy
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
| Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: +49 2137 955 955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλáδα Janssen-Cilag Φαρμακευτικ? Α.Ε.Β.Ε. Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
| Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom (Northern Ireland) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Date of the Last Revision of this Leaflet: MM/AAAA.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AKEEGA 100 MG/500 MG FILM-COATED TABLETSDosage form: TABLET, 50 mg/500 mgActive substance: niraparib and abirateroneManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: TABLET, 100 mgActive substance: olaparibManufacturer: Astrazeneca AbPrescription requiredDosage form: TABLET, 150 mgActive substance: olaparibManufacturer: Astrazeneca AbPrescription required
Online doctors for AKEEGA 100 MG/500 MG FILM-COATED TABLETS
Discuss questions about AKEEGA 100 MG/500 MG FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








