
AKANTIOR 0.8 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS

How to use AKANTIOR 0.8 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
AKANTIOR 0.8 mg/ml eye drops, solution in single-dose container
polyhexanide
Read the entire package leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is AKANTIOR and what is it used for
- What you need to know before you start using AKANTIOR
- How to use AKANTIOR
- Possible side effects
- Storage of AKANTIOR
- Contents of the pack and further information
1. What is AKANTIOR and what is it used for
AKANTIOR contains the active substance polyhexanide.
AKANTIOR is used in adults and children from 12 years of age to treat Acanthamoeba keratitis. Acanthamoeba is a parasite (a tiny organism that lives inside humans and can cause disease) that can cause an infection leading to keratitis (inflammation of the cornea, the transparent layer on the front of the eye). Acanthamoeba keratitis can cause serious defects in the corneal surface, including ulcers (open sores).
AKANTIOR damages the membrane (outer skin) of the Acanthamoeba parasite and causes the release of cellular contents, which destroys the cell. AKANTIOR also prevents the Acanthamoeba parasite from making copies of its DNA by interfering with enzymes (proteins) responsible for the replication process, which disrupts the growth and reproduction of the parasite in humans.
2. What you need to know before you start using AKANTIOR
Do not use AKANTIOR
If you are allergic to polyhexanide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before starting to use AKANTIOR.
Treatment with AKANTIOR may cause mild to moderate eye discomfort (such as eye pain) and red eye. If you experience a severe eye reaction, contact your doctor.
Children and adolescents
AKANTIOR is not recommended for use in children under 12 years of age, as it has not been studied in this age group.
Other medicines and AKANTIOR
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are using another eye drop,wait at least 5 minutes between applying AKANTIOR and applying the other eye drop. AKANTIOR should be applied last.
Pregnancy and breastfeeding
There is no experience with the use of AKANTIOR in pregnant women. AKANTIOR should not be used during pregnancy. If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
It is not known whether AKANTIOR is excreted in breast milk. Ask your doctor or pharmacist for advice before starting treatment with AKANTIOR.
Driving and using machines
Your vision may be temporarily blurred after using AKANTIOR. Do not drive or use machines until your vision is clear again.
AKANTIOR contains phosphates
This medicine contains 0.37 mg of phosphates in each drop, equivalent to 10.66 mg/ml. If you have severe damage to the transparent layer on the front of the eye (cornea), treatment with phosphates may, in very rare cases, cause cloudy patches on the cornea due to calcium.
3. How to use AKANTIOR
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, talk to your doctor again.
The treatment consists of two parts: intensive treatment for the first 19 days and continuation treatment from day 20.
The recommended dose is 1 dropof AKANTIOR in the affected eye as follows:
Initial intensive treatment (19 days)
- Apply one drop every hour (16 times a day) for the first five days (days 1-5)
- Apply one drop every 2 hours (8 times a day) for the next seven days (days 6-12)
- Apply one drop every 3 hours (6 times a day) for the next seven days (days 13-19)
Continuation treatment
- Apply one drop every 4 hours (4 times a day) until there is no corneal inflammation or signs of infection (the eye is healed).
Your doctor will tell you when to stop treatment.
Instructions for use
- Wash your hands.
- Open the aluminum pouch containing the single-dose containers.
- Separate the single-dose container from the strip and place the unopened containers back in the pouch.
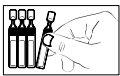
- Open the single-dose container by twisting the top without pulling it. Do not touch the tip after opening the container.
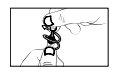
- Tilt your head back. The container is now open. Hold the single-dose container in an upright position and do not press it.
- Use a finger to gently pull down your lower eyelid of the affected eye.
- Invert the single-dose container and place the tip of the container close to your eye. Do not touch the eye or eyelid with the tip of the container.

- Press the single-dose container to administer only one drop, and then release the lower eyelid.
- Close your eye and press a finger on the inner corner (nasal) of the affected eye. Hold the pressure for 2 minutes.
- Discard the single-dose container after use.
If you use more AKANTIOR than you should
Apply the next dose at the usual time, as it is unlikely to cause serious harm.
If you forget to use AKANTIOR
Apply the next dose at the usual time. Do not use a double dose to make up for forgotten doses.
If you stop using AKANTIOR
Use AKANTIOR as your doctor has told you to get the most benefit. Always talk to your doctor if you think you want to stop treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are mild and temporary.
See your doctorif you have a severe eye reaction.
The following side effects have been reported:
Very common(may affect more than 1 in 10 people)
- eye pain
- ocular hyperemia (red eye)
Common(may affect up to 1 in 10 people)
- corneal perforation (damage to the corneal surface)
- visual impairment
- ulcerative keratitis (inflammation or infection of the cornea)
- corneal epithelial defects (defects in the outer layer of the cornea)
- corneal infiltrates (immune response to corneal injury)
- punctate keratitis (small tears in the eye surface)
- tearing (watery eyes)
- conjunctival hyperemia (redness of the conjunctiva)
- ocular inflammation
- ocular irritation
- photophobia (eye sensitivity to light)
- conjunctival papillae (redness, swelling, and irritation of the inner eyelid)
- ocular pruritus (itchy eyes)
- ocular discharge
- ocular edema
- feeling of a foreign body in the eye
- eye discomfort
- dry eye
- conjunctivitis (inflammation of the outer eye layer)
- ocular infection
- worsening of the condition (disease worsening)
- intolerance to the product (hypersensitivity to the medicine)
- reactions at the application site such as pain
- reactions at the application site such as discomfort
- reactions at the application site such as pruritus (itching)
- persistent epithelial defect (persistent loss of the outer corneal layer after injury)
- toxicity to various agents
- need for corneal transplant
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of AKANTIOR
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date is the last day of the month shown on the unopened product.
This medicine does not require any special storage conditions.
After opening the pouch, the single-dose containers must be used within 28 days. After this period, unused single-dose containers should be discarded.
The contents of the single-dose container should be used immediately after opening the container, and any remaining contents should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of AKANTIOR
- The active substance is polyhexanide. Each milliliter of solution contains 0.8 mg of polyhexanide.
- The other ingredients are sodium dihydrogen phosphate monohydrate, disodium phosphate dodecahydrate, sodium chloride, and purified water.
AKANTIOR contains phosphates (see section 2).
Appearance and packaging of the product
AKANTIOR eye drops, solution in single-dose container (eye drops) is a clear, colorless solution in a single-dose container.
The single-dose containers are packaged in sealed strips of 5 units, which are included in a polyester/aluminum/polyethylene pouch and packaged in a box.
Container sizes:
- 20 single-dose containers
- 30 single-dose containers
- multiple container pack containing 120 (4 packs of 30) single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
SIFI S.p.A.
Via Ercole Patti, 36
95025 Aci Sant'Antonio (CT)
Italy
Date of last revision of this package leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
You can access detailed and up-to-date information about this medicine by scanning the QR code included in the package leaflet and on the box with your smartphone or device. You can also access this information at the following internet address: https://qr.sifigroup.com/akantior/.
[QR code]
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AKANTIOR 0.8 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERSDosage form: EYE DROP, 50 mg/mlActive substance: povidone-iodineManufacturer: Alfa Intes Industria Terapeutica Splendore S.R.L.Prescription requiredDosage form: OPHTHALMIC OINTMENT, 20 mg/gActive substance: bibrocatholManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for AKANTIOR 0.8 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Discuss questions about AKANTIOR 0.8 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















