
AFSTYLA 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use AFSTYLA 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
AFSTYLA 250 UI, powder and solvent for solution for injection
AFSTYLA 500 UI, powder and solvent for solution for injection
AFSTYLA 1,000 UI, powder and solvent for solution for injection
AFSTYLA 1,500 UI, powder and solvent for solution for injection
AFSTYLA 2,000 UI, powder and solvent for solution for injection
AFSTYLA 2,500 UI, powder and solvent for solution for injection
AFSTYLA 3,000 UI, powder and solvent for solution for injection
lonoctocog alfa (recombinant single-chain coagulation factor VIII)
Read all of this leaflet carefully before you or your child start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you or your child only. Do not pass it on to others, even if they have the same symptoms as you, as it may harm them.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What AFSTYLA is and what it is used for
- What you need to know before you or your child start using AFSTYLA
- How to use AFSTYLA
- Possible side effects
- Storage of AFSTYLA
- Contents of the pack and further information
1. What AFSTYLA is and what it is used for
AFSTYLA is a product containing human coagulation factor VIII produced by recombinant DNA technology. The active substance of AFSTYLA is lonoctocog alfa.
AFSTYLA is used to treat and prevent bleeding episodes in patients with haemophilia A (congenital factor VIII deficiency). Factor VIII is a protein necessary for blood clotting. Patients with haemophilia A lack this factor, which means their blood does not clot as quickly as it should, and they have a greater tendency to bleed. AFSTYLA works by replacing the missing factor VIII in patients with haemophilia A, allowing their blood to clot normally.
AFSTYLA can be used in all age groups.
2. What you need to know before you start using AFSTYLA
Do not use AFSTYLA
- If the patient has experienced a potentially life-threatening allergic reaction to AFSTYLA or any of its components (listed in section 6).
- If the patient is allergic to hamster proteins.
Warnings and precautions
Traceability
It is important to keep a record of the batch number of AFSTYLA.
Therefore, each time you use a new pack of AFSTYLA, note the date and batch number (which is on the carton after "Batch") and keep this information in a safe place.
Talk to your doctor, pharmacist, or nurse before starting treatment with AFSTYLA.
- Allergic reactions (hypersensitivity) may occur. The product contains residual hamster proteins (see also "Do not use AFSTYLA"). If you experience symptoms of an allergic reaction, stop treatment immediately and contact your doctor.Your doctor should inform you about the first signs of allergic reactions. These include hives, generalised urticaria, chest tightness, difficulty breathing, low blood pressure, and anaphylaxis (a severe allergic reaction that causes severe breathing difficulties and dizziness).
- The formation of inhibitors(antibodies) is a known complication that can occur during treatment with all factor VIII medicines. These inhibitors, especially in large quantities, prevent the treatment from working properly. You or your child will be closely monitored for the development of inhibitors. If your bleeding or your child's bleeding is not being controlled with AFSTYLA, talk to your doctor immediately.
- If you have been told that you or your child have a heart condition or are at risk of having one, inform your doctor or pharmacist.
- If a central venous access device (CVAD) is used for the injection of AFSTYLA, your doctor should consider and discuss with you the risk of complications, such as local infections, bacteria in the blood (bacteraemia), and the formation of blood clots (thrombosis) in the blood vessels at the insertion site.
Other medicines and AFSTYLA
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- During pregnancy and breastfeeding, AFSTYLA should only be used if clearly necessary.
Driving and using machines
AFSTYLA does not affect your ability to drive or use machines.
AFSTYLA contains sodium
This medicine contains 35 mg of sodium (a major component of cooking/table salt) in each vial. This is equivalent to 1.8% of the maximum recommended daily sodium intake for an adult.
3. How to use AFSTYLA
Your treatment should be supervised by a doctor experienced in the treatment of blood clotting disorders.
Follow exactly the administration instructions of this medicine given by your doctor. If in doubt, consult your doctor again.
Dose
The amount of AFSTYLA you or your child need and the duration of treatment depend on:
- the severity of your condition
- the location and intensity of the bleeding
- your clinical condition and clinical response
- your body weight
Follow the instructions given by your doctor.
Reconstitution and administration
General instructions
- The powder should be mixed with the solvent (liquid) and withdrawn from the vial under aseptic conditions.
- AFSTYLA should not be mixed with other medicines or solvents, except those mentioned in section 6.
- The solution should be clear or slightly opalescent, between yellow and colourless, i.e., it may shine when exposed to light but should not contain any visible particles. After filtering or withdrawing the solution (see below), it should be inspected again before use. Do not use the solution if it is visibly cloudy or contains flakes or particles.
- Disposal of unused product and all residual materials should be done in accordance with local regulations and the instructions of your doctor.
Reconstitution and administration
Without opening any of the vials, make sure the AFSTYLA powder and liquid are at room temperature or body temperature. This can be done by leaving the vials at room temperature for about an hour or by holding them in your hands for a few minutes. Do not expose the vials to direct heat. The vials should not be heated above body temperature (37°C).
Carefully remove the protective caps from the vials and then clean the exposed part of the rubber stoppers with an alcohol swab. Let the vials dry before opening the Mix2Vial package (which contains the transfer device with filter) and then follow the instructions below.
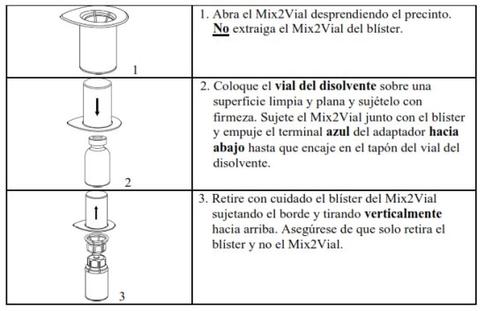

Use the venipuncture kit supplied with the product and insert the needle into a vein. Let the blood flow until the end of the tube. Attach the syringe to the threaded end of the venipuncture kit. Inject the reconstituted solution slowly (at a rate that is comfortable for you, up to a maximum of 10 ml/min) into the veinaccording to the instructions given by your doctor. Try to prevent blood from entering the syringe containing the product.
Check if you experience side effects immediately after injection. If you experience any side effect that may be related to the administration of AFSTYLA, the injection should be interrupted (see also section 2).
Use in children and adolescents
AFSTYLA can be used in children and adolescents of all ages. In the case of children under 12 years, higher doses or more frequent injections may be needed. In children over 12 years, the same dose as for adults can be used.
If you use more AFSTYLA than you should
If more AFSTYLA has been injected than should have been, inform your doctor.
If you forget to use AFSTYLA
Do not administer a double dose to make up for the forgotten dose. Administer the next dose immediately and follow your doctor's instructions.
If you stop treatment with AFSTYLA
If you stop using AFSTYLA, you may no longer be protected against bleeding or may not stop bleeding if you are currently bleeding. Do not stop using AFSTYLA without consulting your doctor first.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, AFSTYLA can cause side effects, although not everybody gets them.
Stop using the medicine immediately and contact your doctor:
- if you notice symptoms of allergic reactions
- Allergic reactions may occur, including the following symptoms: hives, generalised urticaria, chest tightness, difficulty breathing, low blood pressure, and anaphylaxis (a severe allergic reaction that causes severe breathing difficulties and dizziness). If this happens, you should stop the medicine immediately and contact your doctor.
- if you notice that the medicine has stopped working properly(bleeding does not stop) For children who have not been previously treated with factor VIII medicines, inhibitor antibodies (see section 2) may form very frequently (more than 1 in 10 patients); however, in patients who have received previous treatment with factor VIII (more than 150 days of treatment), the risk is uncommon (less than 1 in 100 patients). If you or your child have developed an inhibitor due to the medicine, you may experience persistent bleeding. If this happens, you should contact your doctor immediately.
Common side effects (may affect up to 1 in 10 people)
- Tingling or numbness (paraesthesia).
- Rash.
- Fever.
Uncommon side effects (may affect up to 1 in 100 people)
- Itching.
- Redness of the skin.
- Pain at the injection site.
- Chills.
- Feeling of heat.
Side effects in children and adolescents
No specific age-related differences in adverse reactions have been observed between children, adolescents, and adults.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of AFSTYLA
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and carton.
- Store in a refrigerator (between 2°C and 8°C).
- Before reconstituting the AFSTYLA powder, it can be stored at room temperature (below 25°C) for a single period not exceeding 3 months, within the expiry date printed on the cartons and vials. Note the date when you start storing AFSTYLA at room temperature on the carton of the medicine.
- Once the medicine has been removed from the refrigerator, it must not be put back.
- Do not freeze.
- Keep the vial in its carton to protect it from light.
- Once reconstituted, the medicine should be used immediately.
- If the reconstituted product is not administered immediately, the storage times and conditions before use are the responsibility of the user.
6. Container Contents and Additional Information
Composition of AFSTYLA
The active ingredient is:
250 IU per vial; after reconstitution with 2.5 ml of water for injectable preparations, the solution contains 100 IU/ml of lonoctocog alfa.
500 IU per vial; after reconstitution with 2.5 ml of water for injectable preparations, the solution contains 200 IU/ml of lonoctocog alfa.
1,000 IU per vial; after reconstitution with 2.5 ml of water for injectable preparations, the solution contains 400 IU/ml of lonoctocog alfa.
1,500 IU per vial; after reconstitution with 5 ml of water for injectable preparations, the solution contains 300 IU/ml of lonoctocog alfa.
2,000 IU per vial; after reconstitution with 5 ml of water for injectable preparations, the solution contains 400 IU/ml of lonoctocog alfa.
2,500 IU per vial; after reconstitution with 5 ml of water for injectable preparations, the solution contains 500 IU/ml of lonoctocog alfa.
3,000 IU per vial; after reconstitution with 5 ml of water for injectable preparations, the solution contains 600 IU/ml of lonoctocog alfa.
The other components are:
L-histidine, polysorbate 80, calcium chloride dihydrate, sodium chloride (see the last section of section 2), sucrose.
Solvent: water for injectable preparations.
Appearance of AFSTYLA and Container Contents
AFSTYLA is presented as a white or slightly yellowish powder or friable mass and a clear and colorless solvent for injectable solution.
The reconstituted solution should be transparent or slightly opalescent, between yellow and colorless, i.e., it may shine when exposed to light but should not contain any visible particles.
Presentation
A container with 250, 500, or 1,000 IU containing:
1 vial with powder
1 vial with 2.5 ml of water for injectable preparations
1 transfer device with 20/20 filter
An inner box containing:
1 disposable 5 ml syringe
1 venipuncture device
2 alcohol-impregnated swabs
1 non-sterile dressing
A container with 1,500, 2,000, 2,500, or 3,000 IU containing:
1 vial with powder
1 vial with 5 ml of water for injectable preparations
1 transfer device with 20/20 filter
An inner box containing:
1 disposable 10 ml syringe
1 venipuncture device
2 alcohol-impregnated swabs
1 non-sterile dressing
Only some container sizes may be marketed.
Primary Packaging
250 IU | Glass vial with rubber stopper, orange plastic disc, and green striped aluminum cap |
500 IU | Glass vial with rubber stopper, blue plastic disc, and green striped aluminum cap |
1,000 IU | Glass vial with rubber stopper, green plastic disc, and green striped aluminum cap |
1,500 IU | Glass vial with rubber stopper, turquoise plastic disc, and green striped aluminum cap |
2,000 IU | Glass vial with rubber stopper, purple plastic disc, and green striped aluminum cap |
2,500 IU | Glass vial with rubber stopper, light gray plastic disc, and green striped aluminum cap |
3,000 IU | Glass vial with rubber stopper, yellow plastic disc, and green striped aluminum cap |
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Straße 76
35041 Marburg
Germany
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien CSL Behring NV Tel: +32 15 28 89 20 | Lietuva CentralPharma Communications UAB Tel: +370 5 243 0444 |
| Luxembourg/Luxemburg CSL Behring NV Tel: +32 15 28 89 20 |
Ceská republika CSL Behring s.r.o. Tel: + 420 702 137 233 | Magyarország CSL Behring Kft. Tel.: +36 1 213 4290 |
Danmark CSL Behring AB Tlf: +46 8 544 966 70 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Deutschland CSL Behring GmbH Tel: +49 6190 75 84810 | Nederland CSL Behring BV Tel: + 31 85 111 96 00 |
Eesti CentralPharma Communications OÜ Tel: +3726015540 | Norge CSL Behring AB Tlf: +46 8 544 966 70 |
Ελλάδα CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Österreich CSL Behring GmbH Tel: +43 1 80101 1040 |
España CSL Behring S.A. Tel: +34 933 67 1870 | Polska CSL Behring Sp.z o.o. Tel: +48 22 213 22 65 |
France CSL Behring S.A. Tél: + 33 –(0)-1 53 58 54 00 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
Hrvatska Marti Farm d.o.o. Tel: +385 1 5588297 | România Prisum Healthcare S.R.L. Tel: +40 21 322 0171 |
Ireland CSL Behring GmbH Tel: +49 6190 75 84700 Ísland CSL Behring AB Sími: +46 8 544 966 70 | Slovenija Emmes Biopharma Global s.r.o. podružnica v Sloveniji Tel:+ 386 41 42 0002 Slovenská republika CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Italia CSL Behring S.p.A. Tel: +39 02 34964 200 | Suomi/Finland CSL Behring AB Puh/Tel: +46 8 544 966 70 |
Κύπρος CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Sverige CSL Behring AB Tel: +46 8 544 966 70 |
Latvija CentralPharma Communications SIA Tel: +371 6 7450497 | |
Date of the Last Revision of this Leaflet:{MM/AAAA}
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
----------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Treatment Monitoring
During the course of treatment, it is recommended to properly monitor the factor VIII levels to determine the dose to be administered and the frequency of injections. Patient responses to factor VIII may vary, which demonstrates that they have different half-lives and recoveries. The dose based on body weight may need to be adjusted in patients with insufficient or excessive weight. In the special case of major surgical interventions, it is essential to precisely control the substitution therapy through coagulation analysis (plasma factor VIII activity).
When using a one-stage coagulation assay based on activated partial thromboplastin time (APTT) in vitroto determine the factor VIII activity in patient blood samples, the results of the plasma factor VIII activity may be significantly affected by both the type of APTT reagent and the reference standard used in the assay. Significant discrepancies may also occur between the results obtained in the one-stage coagulation assay based on APTT and those obtained in the chromogenic assay according to the European Pharmacopoeia. This is especially important when changing the laboratory or reagents used in the assay.
The plasma factor VIII activity in patients receiving AFSTYLA with a chromogenic assay or a one-stage coagulation assay should be monitored to guide the administered dose and the frequency of repeated injections. The result of the chromogenic assay more accurately reflects the clinical hemostatic potential of AFSTYLA, so it is the preferred method. The result of the one-stage coagulation assay underestimates the factor VIII activity level compared to the result of the chromogenic assay by approximately 45%. If a one-stage coagulation assay is used, the result is multiplied by a conversion factor of 2 to determine the patient's factor VIII activity level.
Dosage
The dose and duration of substitution therapy depend on the severity of the factor VIII deficiency, the location and extent of the hemorrhage, and the patient's clinical condition.
The number of factor VIII units administered is expressed in International Units (IU), which correspond to the current WHO standard for factor VIII concentrates. Plasma factor VIII activity is expressed as a percentage (in relation to normal human plasma) or, preferably, in International Units (in relation to an international standard for factor VIII in plasma).
One International Unit (IU) of factor VIII activity is equivalent to the amount of factor VIII present in 1 ml of normal human plasma.
Potency assignment is determined by a chromogenic substrate assay.
Plasma factor VIII levels can be monitored using a chromogenic substrate assay or a one-stage coagulation assay.
On-demand Treatment
The calculation of the required dose of factor VIII is based on the empirical finding that 1 International Unit (IU) of factor VIII per kg of body weight increases the plasma activity of factor VIII by 2 IU/dl.
The required dose is determined using the following formula: Dose (IU) = body weight (kg) x desired increase in factor VIII (IU/dl or % of normal level) x 0.5 (IU/kg per IU/dl)
The dose and frequency of administration will always be determined based on the observed clinical efficacy in each case.
In the case of the following hemorrhagic events, the factor VIII activity should not be lower than the established plasma activity level (in % of normal level or IU/dl) during the corresponding period. The following table can be used as a dosage guide in hemorrhagic episodes and surgery:
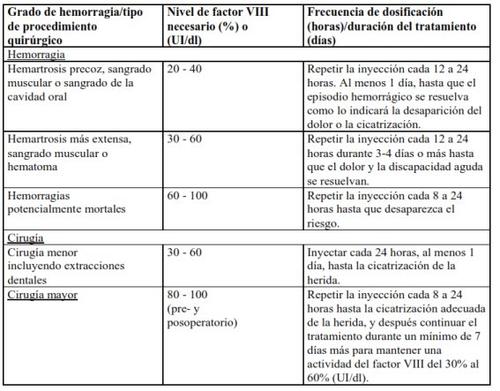
Prophylactic Treatment
The recommended initial treatment regimen is 20 to 50 IU/kg of AFSTYLA administered 2 or 3 times a week. The regimen can be adjusted based on the patient's response.
Pediatric Population
The recommended initial treatment regimen in children (from 0 to <12 years of age) is 30 to 50 iu per kg afstyla administered 2 or 3 times a week. more frequent higher doses may be required in children <12 age due the clearance this group.< p>
In adolescents with 12 years of age or older, the recommended doses are the same as for adults.
Elderly Population
Patients over 65 years of age were not included in the clinical studies of AFSTYLA.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AFSTYLA 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for AFSTYLA 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about AFSTYLA 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















