
ADVATE 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use ADVATE 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ADVATE250UI powder andsolvent for solution for injection
ADVATE500UIpowder andsolvent for solution for injection
ADVATE1000UIpowder andsolvent for solution for injection
ADVATE1500UIpowder andsolvent for solution for injection
ADVATE2000UIpowder andsolvent for solution for injection
ADVATE3000UIpowder andsolvent for solution for injection
Octocog alfa (recombinant human coagulation factor VIII)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is ADVATE and what is it used for
- What you need to know before you use ADVATE
- How to use ADVATE
- Possible side effects
- Storage of ADVATE
- Contents of the pack and further information
1. What is ADVATE and what is it used for
ADVATE contains the active substance octocog alfa, which is a recombinant human coagulation factor VIII produced by DNA technology. Factor VIII is necessary for blood to clot and stop bleeding. In patients with hemophilia A, factor VIII is missing or does not work properly (hereditary absence of factor VIII).
ADVATE is used to treat and prevent bleeding in patients of all ages with hemophilia A (a hereditary bleeding disorder caused by the absence of factor VIII).
ADVATE is prepared without adding any human or animal-derived protein during the entire manufacturing process.
2. What you need to know before you use ADVATE
Do not use ADVATE
- if you are allergic (hypersensitive) to octocog alfa or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to mouse or hamster proteins
If you are not sure if you are allergic, consult your doctor.
Warnings and precautions
Consult your doctor before starting treatment with ADVATE. Inform your doctor if you have been previously treated with medicines containing factor VIII, especially if you developed inhibitors, as there may be a high risk that this will happen again. Inhibitors are antibodies that block factor VIII, reducing the effectiveness of ADVATE in preventing bleeding control. The development of inhibitors is a known complication in the treatment of hemophilia A. If your bleeding is not controlled with ADVATE, consult your doctor immediately.
There is a small risk that you may experience a severe allergic reaction (anaphylaxis) to ADVATE. You should be aware that the first symptoms of an allergic reaction are rash, itching, hives, generalized itching, swelling of the lips and tongue, difficulty breathing, wheezing, chest tightness, feeling of general discomfort, and dizziness. These symptoms can be a warning of anaphylactic shock, which can also cause severe dizziness, loss of consciousness, and severe breathing difficulties.
If you experience any of these symptoms, stop the administration of the medicine immediately and consult a doctor. Severe symptoms, including difficulty breathing and (near) fainting, require rapid emergency treatment.
Patients who develop factor VIII inhibitors
If the factor VIII level in your plasma does not reach the expected values with ADVATE, or if bleeding is not adequately controlled, it may be due to the development of factor VIII inhibitors. This will be checked by your doctor. You may need a higher dose of ADVATE or even another medicine to control bleeding. Do not increase the total dose of ADVATE you use to control your bleeding without consulting your doctor.
Children and adolescents
The warnings and precautions mentioned apply to adults and children (from 0 to 18 years of age).
Using ADVATE with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy andbreast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving andusing machines
Treatment with ADVATE does not affect the ability to drive and use machines.
ADVATE contains sodium
This medicine contains 0.45 mmol of sodium (10 mg) per vial, which should be taken into account in patients on a low-sodium diet.
3. How to use ADVATE
Treatment with ADVATE will be started by a doctor with experience in treating patients with hemophilia A.
Your doctor will calculate your dose of ADVATE (in international units or IU) based on your condition, body weight, and whether it is to be used for prevention or treatment of bleeding. The frequency of administration will depend on how ADVATE works. Replacement therapy with ADVATE is usually a lifelong treatment.
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are unsure, consult your doctor again.
Prevention of bleeding
The usual dose of octocog alfa is 20 to 40 IU per kilogram of body weight, administered every 2 or 3 days. However, in some cases, especially in younger patients, more frequent administration of injections or higher doses may be required.
Treatment of bleeding
The dose of octocog alfa is calculated based on your body weight and the desired factor VIII levels. The factor VIII levels to be achieved will depend on the severity and location of the bleeding.
Dose (IU) = body weight (kg) x desired increase in factor VIII (% of normal) x 0.5
If you think the effect of ADVATE is too weak, consult your doctor.
Your doctor will perform the necessary laboratory tests to ensure you have adequate factor VIII levels. This is especially important if you are going to undergo major surgery.
Use in children and adolescents (from0to18years of age)
For the treatment of bleeding, the dose in children does not differ from that in adult patients. To prevent bleeding in children under 6 years of age, doses of 20 to 50 IU per kilogram of body weight are recommended 3 to 4 times a week. The administration of ADVATE in children (intravenously) does not differ from administration in adults. A central venous access device (CVAD) may be necessary to allow frequent infusions of factor VIII products.
How ADVATE is administered
ADVATE is usually injected into a vein (intravenously) by your doctor or nurse. You or another person can also administer the injection of ADVATE, but only after receiving proper training. Detailed instructions for administration are described at the end of this leaflet.
If you use more ADVATE than you should
Follow the instructions for administration of ADVATE exactly as your doctor has told you. Consult your doctor if you have any doubts. If you inject a higher dose of ADVATE than recommended, consult your doctor as soon as possible.
If you forget to use ADVATE
Do not inject a double dose to make up for forgotten doses. Administer the next injection as scheduled and continue as your doctor has told you.
If you stop treatment with ADVATE
Do not stop using ADVATE without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience sudden and severe allergic reactions(anaphylaxis), stop the injection immediately. Contact your doctor immediately if you have any of the following initial symptoms of allergic reactions:
- rash, itching, hives, and generalized itching,
- swelling of the lips and tongue,
- difficulty breathing, wheezing, chest tightness,
- feeling of general discomfort,
- dizziness and loss of consciousness.
Severe symptoms, such as difficulty breathing and (near) fainting, require early emergency treatment.
Common side effects(may affect up to 1 in 10 people)
factor VIII inhibitors, headache, and fever
Uncommon side effects(may affect up to 1 in 100 people)
dizziness, flu-like symptoms, fainting, irregular heartbeats, red spots associated with itching, chest discomfort, injection site reaction, itching, increased sweating, unusual taste in the mouth, hot flushes, migraines, memory loss, chills, diarrhea, nausea, vomiting, difficulty breathing, sore throat, infection of the lymphatic vessels, pallor, eye inflammation, rash, excessive sweating, inflammation of feet and ankles, decreased red blood cell count, increased monocytes (a type of white blood cell), and upper abdominal or lower chest pain.
Related to surgery
catheter-related infection, decreased red blood cell count, swelling of the limbs and joints, prolonged bleeding after drain removal, decreased factor VIII levels, and postoperative hematoma
Related to central venous access devices (CVAD)
Catheter-related infection, systemic infection, and local blood clot at the catheter site.
Side effects with unknown frequency(cannot be estimated from the available data)
potentially life-threatening reactions (anaphylaxis) and other allergic reactions (hypersensitivity), general disorders (fatigue, lack of energy).
Additional side effects in children
In addition to the development of inhibitors in previously untreated pediatric patients and catheter-related complications, no age-specific differences in side effects were observed in clinical trials.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ADVATE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month stated.
Store in a refrigerator (2°C to 8°C).
Do not freeze.
Until the expiry date, the blister with the medicine can be stored at room temperature (up to 25°C) for a single period not exceeding 6 months. In this case, this medicine expires at the end of this 6-month period or on the expiry date printed on the blister, whichever is earlier. Please note the end date of the 6-month storage period at room temperature on the medicine carton. The medicine cannot be refrigerated again after storage at room temperature.
Store the blister with the medicine in the outer carton to protect it from light.
This medicine is for single use only. Dispose of any unused solution properly.
Use the medicine immediately after complete dissolution of the powder.
Do not refrigerate the medicine after preparation.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition of ADVATE
- The active ingredient is octocog alfa (human coagulation factor VIII produced by recombinant DNA technology). Each vial of powder contains nominally 250, 500, 1000, 1500, 2000, or 3000 IU of octocog alfa.
- The other components are mannitol, sodium chloride, histidine, trehalose, calcium chloride, trometamol, polysorbate 80, and glutathione (reduced)
Solvent vial: 5 ml of sterile water for injectable preparations
Appearance of the Product andContainer Contents
ADVATE is a white to off-white, friable powder.
After reconstitution, the solution is clear, colorless, and free of foreign particles.
Marketing Authorization Holder
Baxter AG
Industriestrasse 67
A-1221 Vienna
Manufacturers
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B-7860 Lessines
Belgium
Baxter SA
Boulevard René Branquart 80
B-7860 Lessines
Belgium
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Baxalta Belgium SPRL Tel: +32 2 892 62 00 | Lietuva UAB "Baxter Lithuania" Tel: +370 5 269 16 90 / +370 5 252 71 00 |
България Бакстер България ЕООД Tel: +359 2 9808482 | Luxembourg/Luxemburg Baxalta Belgium SPRL Tel: +32 2 892 62 00 |
Ceská republika Baxter Czech spol.s.r.o. Tel: +420 225774111 | Magyarország Baxter Hungary Kft. Tel: +36 1 202 1980 |
Danmark Baxalta Denmark A/S Tlf: +45 32 70 12 00 | Malta Baxalta UK Limited Tel: +44 1 635 798 777 |
Deutschland Baxalta Deutschland GmbH Tel: +49 89 262077-011 | Nederland Baxalta Netherlands B.V. Tel: +31 30 799 27 77 |
Eesti OÜ Baxter Estonia Tel: +372 6 515 120 | Norge Baxalta Norway AS Tlf: +47 22 585 000 |
Ελλάδα Baxter (Hellas) Ε.Π.Ε. Τηλ: +30 210 28 80 000 | Österreich Baxalta Österreich GmbH Tel: +43 1 20100-0 |
España Baxalta Spain S.L. Tel: +34 91 790 42 22 | Polska Baxter Polska Sp. z o.o. Tel: +48 22 4883 777 |
France Baxalta France S.A.S. Tel: +33 1 70 96 06 00 | Portugal Baxalta Portugal, Unipessoal, Lda. Tel: +351 21 122 03 00 |
Hrvatska Baxter d.o.o. Tel: +386 1 420 16 80 | România FARMACEUTICA REMEDIA SA Tel: +40 21 321 16 40 |
Ireland Baxalta UK Limited Tel: +44 1 635 798 777 | Slovenija Baxter d.o.o. Tel: +386 1 420 16 80 |
Date of Last Revision of this Leaflet
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑
Instructions for Preparation andAdministration
Aseptic technique is required for the preparation of the solution and its administration.
Use only the sterile water for injectable preparations and the reconstitution equipment included in each ADVATE container. ADVATE should not be mixed with other medicinal products or solvents.
It is strongly recommended to record the name and batch number of the product each time ADVATE is administered.
Reconstitution Instructions
- Do not use after the expiry date stated on the labels and carton.
- Do not use if the BAXJECT II device, the sterile protective system, or its packaging is damaged or shows signs of deterioration, as indicated by the symbol: .
- Do not refrigerate the preparation after reconstitution.
- If the medicinal product is stored in the refrigerator, remove the ADVATE powder and solvent vials from the refrigerator and let them reach room temperature (between 15°C and 25°C).
- Wash your hands with soap and warm water.
- Remove the protectors from the powder and solvent vials.
- Clean the stoppers with alcohol-impregnated swabs. Place the vials on a flat, clean surface.
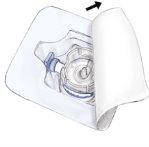
- Open the BAXJECT II device packaging by removing the paper cover without touching the inside (Fig. a). Do not remove the device from the packaging. Do not use if the BAXJECT II device, its sterile barrier system, or its packaging is damaged or shows signs of deterioration.
- Turn the packaging over and insert the plastic tip through the solvent stopper. Hold the packaging by its end and remove the BAXJECT II device from its packaging (Fig. b). Do not remove the blue protector from the BAXJECT II device.
- For reconstitution, use only the sterile water for injectable preparations and the reconstitution equipment included in the container. With the BAXJECT II device attached to the solvent vial, turn the system over so that the solvent vial is on top of the device. Insert the white plastic tip into the ADVATE powder vial stopper. The vacuum will draw the solvent into the ADVATE powder vial (Fig. c).
- Gently swirl until all material is dissolved. Ensure that the ADVATE powder is completely dissolved; if not, the reconstituted solution will not pass through the filter of the device. The medicinal product dissolves rapidly (usually in less than 1 minute). After reconstitution, the solution should be clear, colorless, and free of foreign particles.
Fig. aFig. bFig. c



Instructions for Injection
A luer-lock syringe is required for administration.
Important note:
- Do not attempt to administer the injection unless you have received special training from your doctor or nurse.
- Inspect the prepared solution for particles or discoloration before administration (the solution should be clear, colorless, and free of foreign particles).
Do not use ADVATE if the solution is not totally clear or not completely dissolved.
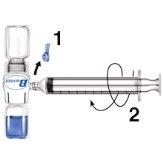
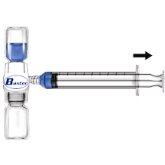
- Remove the blue protector from the BAXJECT II device. Do not introduce air into the syringe. Attach the syringe to the BAXJECT II device (Fig. d).
- Turn the system over (the vial with the reconstituted solution on top). Draw the reconstituted solution into the syringe by slowly pulling back the plunger (Fig. e).
- Disconnect the syringe.
- Attach a winged infusion needle to the syringe. Administer intravenously. The solution should be administered slowly, at a rate determined by the patient's comfort level, not exceeding 10 ml per minute. The pulse should be taken before and during the administration of ADVATE. If a significant increase in pulse rate is observed, the administration rate should be reduced or temporarily interrupted, usually allowing the symptoms to disappear (see section 4 "Possible Side Effects").
- Dispose of unused solution properly.
Fig. dFig. e
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑
This information is intended for healthcare professionals only:
On-demand Treatment
In the case of subsequent hemorrhagic episodes, factor VIII activity should not fall below the given plasma activity level (in % of normal or IU/dl) during the corresponding period. The following table can be used as a guide for dosing in hemorrhagic episodes and surgery.
The dose and frequency of administration should be adapted to the individual clinical response. In certain circumstances (e.g., presence of a low inhibitor titre), higher doses than those calculated using the formula may be necessary.
Severity of Hemorrhage / Type of Surgical Procedure | Required Factor VIII Level (% orIU/dl) | Dose Frequency (hours) / Duration of Therapy (days) |
Hemorrhage Early hemarthrosis or muscle or oral hemorrhage. More extensive hemarthrosis, muscle hemorrhage, or hematoma. Life-threatening hemorrhage. | 20 – 40 30 – 60 60 – 100 | Repeat injection every 12 to 24 hours (every 8 to 24 hours in patients under 6 years of age) for at least 1 day until the hemorrhagic episode, as indicated by pain, is resolved or healing is achieved. Repeat injection every 12 to 24 hours (every 8 to 24 hours in patients under 6 years of age) for 3 to 4 days or more, until pain and acute disability have ceased. Repeat injection every 8 to 24 hours (every 6 to 12 hours in patients under 6 years of age) until the danger has passed. |
Surgery Minor Including dental extraction. Major | 30 – 60 80 – 100 (pre- and post-operative) | Every 24 hours (every 12 to 24 hours in patients under 6 years of age), for at least 1 day, until healing is achieved. Repeat injection every 8 to 24 hours (every 6 to 24 hours in patients under 6 years of age) until adequate wound healing is achieved, and then for at least another 7 days of therapy to maintain a factor VIII activity of 30% to 60% (IU/dl). |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ADVATE 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for ADVATE 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about ADVATE 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















