
Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution

Ask a doctor about a prescription for Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution

How to use Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution
Introduction
Package Leaflet: Information for the Patient
Acetilcisteína Kern Pharma 100 mg/ml Solution for Injection EFG
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Acetilcisteína Kern Pharma and what is it used for
- What you need to know before you use Acetilcisteína Kern Pharma
- How to use Acetilcisteína Kern Pharma
- Possible side effects
- Storage of Acetilcisteína Kern Pharma
- Contents of the pack and other information
1. What is Acetilcisteína Kern Pharma and what is it used for
Acetilcisteína Kern Pharma contains the active substance acetilcisteína, which belongs to a group of medicines called mucolytics that decrease the viscosity of mucus, making it more fluid and easier to eliminate from the respiratory tract.
Acetilcisteína Kern Pharma is indicated to facilitate the elimination of excess mucus and phlegm in adults and children from 2 years of age, in respiratory processes with bronchial hypersecretion such as: Acute and chronic bronchitis, chronic obstructive pulmonary disease (COPD), emphysema, pulmonary complications of cystic fibrosis, facilitation of maneuvers in anesthesia for bronchoscopies, bronchographies and bronchoaspiration, bronchiectasis, obstructive and infectious complications due to tracheotomy and bronchopulmonary due to surgical intervention.
2. What you need to know before you use Acetilcisteína Kern Pharma
Do not use Acetilcisteína Kern Pharma:
- If you are allergic to the active substance or to any of the other components of this medicine (listed in section 6).
- Do not administer to children under 2 years of age.
Warnings and precautions
Consult your doctor or pharmacist before starting to use acetilcisteína.
If you are asthmatic or have a severe respiratory disease, you should consult your doctor before taking this medicine as it may cause respiratory difficulties (bronchospasm).
The possible sulfurous odor (like rotten eggs) of the medicine is characteristic of the active substance, but it does not indicate that its characteristics have been altered.
During the first days of treatment, you may observe an increase in mucus and phlegm, which will decrease throughout the treatment. If you see that you are not able to expectorate effectively, postural drainage and bronchoaspiration should be carried out.
Intravenous administration will be carried out under strict medical supervision. It is more likely that adverse reactions will appear after intravenous perfusion if the drug is administered too quickly or in excess. Therefore, it is recommended to strictly follow the instructions that appear in section 3. How to use Acetilcisteína Kern Pharma.
Acetilcisteína is not compatible with rubber and certain metals, especially iron, nickel, and copper. Contact with materials that contain them should be avoided.
It should be administered with caution in long-term treatment in patients with histamine intolerance.
Children and adolescents
In children and adolescents, the same precautions and warnings apply.
It is contraindicated in children under 2 years of age.
Using Acetilcisteína Kern Pharma with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine.
In case you need simultaneous treatment with nitroglycerin, you should monitor the appearance of hypotension (low blood pressure), which can be severe, and may cause headache.
The simultaneous use of carbamazepine, a drug used to combat epilepsy attacks, may increase the risk of attacks.
Do not administer together with antitussive medicines (for cough) or with those that decrease bronchial secretions (such as antihistamines and anticholinergics), as it could lead to an accumulation of bronchial secretions.
Separate administration of antibiotics is recommended.
It is not recommended to dissolve acetilcisteína with other medicines.
Using Acetilcisteína Kern Pharma with food and drinks
Taking food and drinks does not affect the efficacy of this medicine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Acetilcisteína crosses the placenta. Therefore, the use of acetilcisteína during pregnancy is not recommended.
It is unknown whether acetilcisteína and its metabolites are excreted in breast milk. Its use during breastfeeding should be avoided.
No data are available on the effect of acetilcisteína on human fertility. Animal studies do not indicate harmful effects on human fertility at the recommended doses.
Driving and using machines
There is no evidence of effects on the ability to drive and use machines.
Acetilcisteína Kern Pharma contains sodium
This medicine contains 41.2 mg of sodium (main component of table/cooking salt) in each 3 ml ampoule. This is equivalent to 2.06% of the maximum recommended daily intake of sodium for an adult.
Interference with laboratory tests
Acetilcisteína may interfere with the colorimetric method for the determination of salicylates.
Acetilcisteína may interfere with the ketone test in urine.
3. How to use Acetilcisteína Kern Pharma
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Local administration:
Inhalation via nebulizer
Adults and children from 12 years of age: one 300 mg ampoule one or two times a day for 5 to 10 days.
Children between 2 and 12 years of age: up to one 300 mg ampoule one or two times a day for 5 to 10 days in children who cooperate.
Endotracheobronchial route
Adults and children from 12 years of age: one 300 mg ampoule (60 drops) one or two times a day for 5 to 10 days.
Children between 2 and 12 years of age: up to one 300 mg ampoule (60 drops) one or two times a day for 5 to 10 days.
Parenteral administration:
This medicine can be administered in bronchial conditions when local treatment is impossible or difficult, or when the doctor prefers the systemic route (lack of patient cooperation, mandatory bed rest, closed circuit breathing, etc.).
Intramuscular route
Adults and children from 12 years of age: one 300 mg ampoule one or two times a day administered via deep injection.
Children between 2 and 12 years of age: 150 mg (half 3 ml ampoule) one or two times a day administered via deep injection.
Intravenous route
Acetilcisteína administration via intravenous route is performed under strict medical supervision.
The medicine should be administered via slow perfusion in saline solution or 5% glucose solution.
Adults and children from 12 years of age: one 300 mg ampoule one or two times a day.
Children between 2 and 12 years of age: 150 mg (half 3 ml ampoule) one or two times a day.
Opening the ampoule:
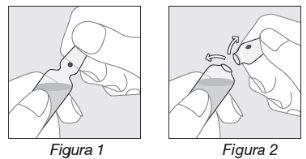
- Hold the ampoule as indicated in Figure 1;
- Place your thumb on the colored point and exert pressure backwards as indicated in Figure 2.
Duration of treatment
The duration of treatment should be established according to clinical evolution. The average duration is 5-10 days. The high general and local tolerability of acetilcisteína allows for prolonged treatments in certain cases.
If you use more Acetilcisteína Kern Pharma than you should
If you have used more acetilcisteína than you should, you may notice symptoms similar to those described in section 4. Possible side effects, although more intense. In case of overdose or accidental massive administration, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91.562.04.20, indicating the medicine and the amount used.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur, although their frequency cannot be established from the available information:
Local use:
Allergic reactions (hypersensitivity), constriction of the bronchi and difficulty breathing (bronchospasm), increased nasal secretion (rhinorrhea), mouth sores (stomatitis), vomiting, nausea, urticaria, rash, or itching.
Parenteral use:
Allergic reactions (hypersensitivity) of various degrees, which can lead to anaphylactic shock, increased heart rate (tachycardia), constriction of the bronchi and difficulty breathing (bronchospasm, dyspnea), vomiting, nausea, facial swelling (angioedema), urticaria, flushing, rash, itching, facial edema, decreased blood pressure, decreased blood coagulation (increased prothrombin time, decreased platelet aggregation).
In very rare cases, severe skin reactions (Stevens-Johnson syndrome and Lyell syndrome) may appear, although in most cases, at least one other suspect drug could be identified as triggering the syndrome.
In case of any alteration in the skin or mucous membranes, acetilcisteína administration should be interrupted immediately. The specialist doctor will determine the treatment to follow.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Acetilcisteína Kern Pharma
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiry date which is stated on the packaging after the abbreviation CAD. The expiry date is the last day of the month indicated.
Local administration
It is recommended to open the ampoule at the time of use. Opened ampoules can only be used for local use and should be stored in the refrigerator for a maximum of 24 hours.
Parenteral administration
Use immediately after opening. If not used immediately, the storage times and conditions are the responsibility of the user.
The solution, once diluted for use (in 5% glucose solution or 0.9% sodium chloride solution), remains stable for 24 hours at 25°C.
Discard after use.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Acetilcisteína Kern Pharma
The active substance is acetilcisteína. Each 3 ml ampoule contains 300 mg of acetilcisteína.
The other components are: disodium edetate, sodium hydroxide (for pH adjustment), and water for injectable preparations.
Appearance of the product and pack contents
Appearance: topaz glass ampoules with a break point, containing 3 ml of a clear and colorless solution.
Each pack contains 5 ampoules.
Marketing authorization holder and manufacturer
Kern Pharma, S.L.
Venus, 72 – Pol. Ind. Colón II
08228 Terrassa - Barcelona
Spain
Date of the last revision of this package leaflet:December 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Availability in pharmaciesSupply issue reported
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Acetylcysteine Kern Pharma 100 mg/ml Injectable SolutionDosage form: EFFERVESCENT TABLET, 600 mgActive substance: acetylcysteineManufacturer: Kern Pharma S.L.Prescription not requiredDosage form: EFFERVESCENT TABLET, 600 mgActive substance: acetylcysteineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: EFFERVESCENT TABLET, 200 mgActive substance: acetylcysteineManufacturer: Aurovitas Spain, S.A.U.Prescription required
Alternatives to Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution in Poland
Alternative to Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution in Ukraine
Online doctors for Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Acetylcysteine Kern Pharma 100 mg/ml Injectable Solution – subject to medical assessment and local rules.














