

Zamidine

Ask a doctor about a prescription for Zamidine

How to use Zamidine
Leaflet attached to the packaging: patient information
Zamidine, 1 mg/ml, eye drops, solution
Hexamidini diisethionas
Please read carefully the contents of the leaflet before using the medicine, as it contains important information for the patient.
- Please keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, please consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. Do not pass it on to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, please tell the doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Zamidine and what is it used for
- 2. Important information before using Zamidine
- 3. How to use Zamidine
- 4. Possible side effects
- 5. How to store Zamidine
- 6. Contents of the packaging and other information
1. What is Zamidine and what is it used for
Zamidine contains an antibacterial antiseptic agent. The medicine is used as a local antiseptic in the treatment of certain eye infections and surrounding structures, such as:
- inflammation of the outer surface of the eye (conjunctivitis),
- inflammation of certain parts of the eye: conjunctiva and cornea (keratoconjunctivitis),
- local inflammation of the eyelid margins, often at the base of the eyelashes (blepharitis),
- chronic inflammation of the lacrimal sacs.
This medicine is also used for the disinfection of the conjunctival sacs (the space between the eyelids and the eyeball) before surgery.
Zamidine is an eye drop solution without preservatives.
2. Important information before using Zamidine
When not to use Zamidine
- if the patient is allergic to hexamidine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use this medicine, please discuss it with a doctor or pharmacist.
Please stop the treatment and contact a doctor if:
- the patient's condition does not improve after using the medicine as recommended by the doctor,
- any new symptoms appear (redness, eye pain or blurred vision). If the patient has an eye infection, do not wear contact lenses during the entire treatment period.
Zamidine and other medicines
Please tell the doctor or pharmacist about all medicines that the patient is currently taking or has recently taken, as well as any medicines that the patient plans to take.
Pregnancy and breastfeeding
Zamidine may be used during pregnancy or breastfeeding.
Driving and using machines
Zamidine does not affect vision, but may cause temporary blurred vision or other vision disturbances that may potentially affect the ability to drive or operate machines. If blurred vision occurs, do not drive or operate machines until normal vision returns.
3. How to use Zamidine
This medicine should always be used exactly as recommended by the doctor or pharmacist. In case of doubts, please consult a doctor or pharmacist.
The recommended dose is one drop into the conjunctival sac (the space between the lower eyelid and the eye) of the affected eye (eyes) 4 to 6 times a day.
Please avoid prolonged or repeated treatment, as bacteria may become resistant to the product. For this reason, do not exceed the treatment duration recommended by the doctor.
Use in children
The safety and efficacy of using these eye drops in children have not been established. There are no available data.
Method and route of administration
This medicine should be administered into the eye.
If this medicine and other eye medicines are used, please wait at least 15 minutes between consecutive instillations.
This medicine is a preservative-free eye drop solution. Please do not allow the tip of the multidose container to touch the eye or the surrounding area. The tip may become contaminated and cause a risk of eye infection. To avoid potential contamination of the multidose container, please keep the tip of the multidose container away from any surface.
For the 0.6 ml multidose container:
To use Zamidine, please follow the instructions below:
- 1. Please wash your hands and take a comfortable standing or sitting position.
- 2. Open the sachet containing 5 multidose containers. Record the date of first opening on the sachet.
- 3. Break off one multidose container from the strip.
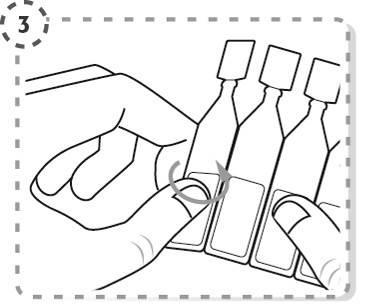
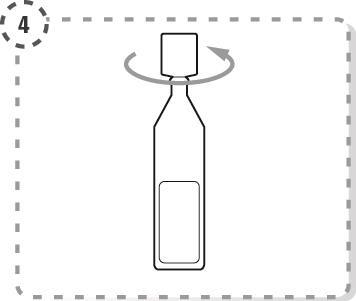
- 4. Unscrew the top of the multidose container, as shown in the picture. Do not touch the tip of the container with your fingers after opening.
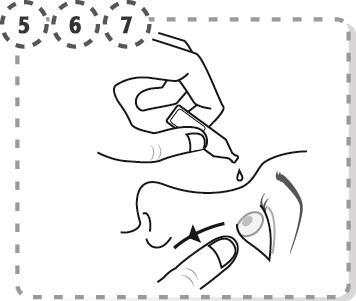
- 5. Gently pull down the lower eyelid of the affected eye.
- 6. Place the tip of the multidose container close to the eye, without touching the eye, eyelashes, fingers, or other surfaces with the dropper.
- 7. Gently squeeze the multidose container to release one drop into the eye, then release the lower eyelid.
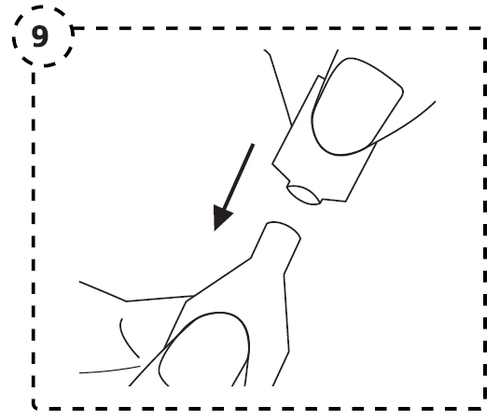
- 8. Repeat the steps for the second eye, if it is affected.
- 9. After use, the multidose container should be closed immediately: tightly press the cap onto the tip of the multidose container, if not all of its contents have been used. Each reclosed multidose container may be reused for up to 24 hours.
- 10. Discard the multidose container after 24 hours of opening or when it is empty.
For the 10 ml multidose container:
To use Zamidine, please follow the instructions below:
- 1. Please wash your hands and take a comfortable standing or sitting position.
- 2. Unscrew the cap to open the multidose container. Do not touch the tip of the container with your fingers after opening.
- 3. Tilt your head back and gently pull down the lower eyelid of the affected eye.
- 4. Place the tip of the multidose container close to the eye, without touching the eye, eyelashes, fingers, or other surfaces with the dropper.
- 5. Gently squeeze the multidose container to release one drop into the eye, then release the lower eyelid.
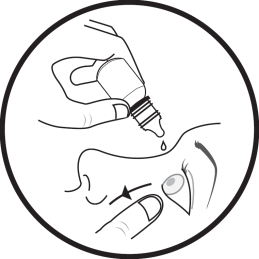
- 6. Repeat the steps for the second eye, if it is affected.
- 7. After use, the multidose container should be closed by screwing the cap.
- 8. Discard the multidose container after 30 days.
Using more than the recommended dose of Zamidine
In case of instilling too much medicine into the eye, please rinse the eye with saline solution.
Missing a dose of Zamidine
Do not use a double dose to make up for a missed dose.
Stopping the use of Zamidine
This medicine should be used exactly as described in this leaflet. If there is no improvement or the patient feels worse, please contact a doctor.
In case of any further doubts about the use of this medicine, please consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur:
Frequency not known (frequency cannot be estimated from the available data).
- Local allergic reaction (eye redness, eyelid swelling and redness, itching).
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, please tell the doctor or pharmacist.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw
tel.: +48 22 49 21 301, fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Zamidine
The medicine should be stored out of sight and reach of children.
For the 0.6 ml multidose container:
Do not use this medicine after the expiry date stated on the carton, sachet, and multidose container. The expiry date refers to the last day of the month.
For the 10 ml multidose container:
Do not use this medicine after the expiry date stated on the carton and multidose container. The expiry date refers to the last day of the month.
Store below 25°C.
For the 0.6 ml multidose container:
After first opening of the sachet: shelf life of the multidose containers: 30 days.Please record the date of first opening on the sachet.
After first opening of the multidose container: shelf life of the reclosed multidose container: 24 hours from the first opening of the multidose container.
For the 10 ml multidose container:
After first opening of the multidose container: shelf life is 30 days from the first opening of the multidose container.
Medicines should not be disposed of via wastewater or household waste. Please ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Zamidine contains
- The active substance is hexamidine diisethionate. 1 ml of solution contains 1 mg of hexamidine diisethionate.
- The other ingredients are: boric acid, borax, sodium chloride, water for injections.
What Zamidine looks like and contents of the packaging
For the 0.6 ml multidose container:
This medicinal product is an eye drop solution. The solution is clear, colorless, and available in multidose containers packaged in sachets of 5 units. Each multidose container contains 0.6 ml of medicine.
Each 0.6 ml multidose container contains at least 12 preservative-free eye drops.
For the 10 ml multidose container:
This medicinal product is an eye drop solution. The solution is clear, colorless, and available in multidose containers of 10 ml.
Each 10 ml multidose container contains approximately 250 preservative-free eye drops.
The packaging contains 5 or 10 (2x5) multidose containers of 0.6 ml or one multidose container of 10 ml.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Laboratoires THEA
12 rue Louis Blériot
63017 Clermont-Ferrand Cedex 2
France
Manufacturer:
For the 0.6 ml multidose container:
Laboratoires THEA
12 rue Louis Blériot
63017 Clermont-Ferrand Cedex 2
France
LABORATOIRE UNITHER
1 rue de l’Arquerie
50200 Coutances
France
For the 10 ml multidose container:
Laboratoires THEA
12 rue Louis Blériot
63017 Clermont-Ferrand Cedex 2
France
To obtain more detailed information, please contact the representative of the marketing authorization holder:
THEA Polska Sp. z o.o.
ul. Cicha 7
00-353 Warsaw
Tel.: +48 22 642 87 77
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium, Luxembourg ..................................................................................................... Zasetic
Bulgaria, Germany, Poland, Romania, Italy ............................................................... Zamidine
Czech Republic, Denmark, Finland, Iceland, Norway, Portugal, Slovakia, Sweden ............ Zamisept
France, Greece ............................................................................................................. Zameline
Date of last revision of the leaflet: 24-05-2024
Detailed information on this medicinal product is available on the website www.urpl.gov.pl
Health education - Advice in case of eye infection
If there is no improvement or the patient feels worse, please contact a doctor.
Since eye infections are contagious, please take the following simple steps to avoid transferring the infection to the other eye or to a family member:
Please wash your hands regularly with warm water and soap
Do not rub your eyes
Wash pillows and face towels in hot water with detergent
Do not use shared towels and pillows
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratoire Unither Laboratoires THEA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZamidineDosage form: Ointment, 20 mg/gActive substance: bibrocatholManufacturer: Ursapharm Arzneimittel GmbHPrescription requiredDosage form: Drops, 10 mg/mlActive substance: silver compoundsPrescription not requiredDosage form: Drops, 50 mg/mlActive substance: povidone-iodineManufacturer: Alfa Intes Industria Terapeutica Splendore S.r.l.Prescription required
Alternatives to Zamidine in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Zamidine in Ukraine
Alternative to Zamidine in Spain
Online doctors for Zamidine
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Zamidine – subject to medical assessment and local rules.














