
Xancodal
Ask a doctor about a prescription for Xancodal

How to use Xancodal
Leaflet accompanying the packaging: patient information
Xancodal, 5 mg, hard capsules
Xancodal, 10 mg, hard capsules
Xancodal, 20 mg, hard capsules
Oxycodone hydrochloride
For use in adults and adolescents from 12 years of age
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for a specific person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Xancodal and what is it used for
- 2. Important information before taking Xancodal
- 3. How to take Xancodal
- 4. Possible side effects
- 5. How to store Xancodal
- 6. Package contents and other information
1. What is Xancodal and what is it used for
Xancodal contains the active substance oxycodone hydrochloride, which is a strong pain reliever from the opioid group.
Xancodal is used in adults and adolescents from 12 years of age to treat severe pain that requires opioid pain relievers.
2. Important information before taking Xancodal
When not to take Xancodal
if the patient is allergic to oxycodone hydrochloride or any of the other ingredients of this medicine (listed in section 6);
if the patient has breathing difficulties, such as severe respiratory depression, chronic obstructive pulmonary disease, or severe asthma. Symptoms may include shortness of breath, coughing, or slower or weaker breathing than normal.
if the patient has increased carbon dioxide levels in the blood;
if the patient has heart changes caused by long-term lung disease (pulmonary heart disease);
if the patient has intestinal obstruction. It may be indicated by slower than normal emptying of the stomach or severe abdominal pain.
Warnings and precautions
Before taking Xancodal, discuss with your doctor or pharmacist if:
- the patient is elderly or frail
- the patient has severe lung function disorders
- the patient has liver or kidney function disorders
- the patient has thyroid disease, dryness, coldness, and swelling of the skin of the face and limbs (myxedema)
- the patient has reduced thyroid function
- the patient has adrenal insufficiency (Addison's disease), which may cause symptoms such as weakness, weight loss, dizziness, nausea, or vomiting
- the patient has an enlarged prostate gland, which causes difficulty urinating (in men)
- stopping alcohol or drug use in the past has caused the patient to experience withdrawal symptoms, such as agitation, anxiety, tremors, or excessive sweating
- or if anyone in your family has ever abused or been dependent on alcohol, prescription drugs, or illegal drugs ("addiction")
- the patient is a smoker
- the patient has ever had mood problems (depression, anxiety, or personality disorders) or has been treated by a psychiatrist for other mental illnesses
- the patient has mental disorders due to poisoning, e.g. with alcohol (toxic psychosis)
- the patient has pancreatitis, which causes severe abdominal and back pain
- the patient has gallbladder or bile duct disorders
- the patient has intestinal disease related to obstruction or inflammation
- the patient has a head injury, severe headache, or feels nauseous, as it may indicate increased intracranial pressure
- the patient has low blood pressure
- the patient has low blood volume (hypovolemia), e.g. due to significant bleeding, severe burns, excessive sweating, severe diarrhea, or vomiting
- the patient has epilepsy or a tendency to seizures/convulsions
- the patient is taking monoamine oxidase inhibitors (MAOIs) (used to treat depression or Parkinson's disease) or has taken them within the last 2 weeks
- the patient is scheduled to have or has recently had abdominal surgery.
If any of the above situations apply to the patient currently or in the past, they should tell their doctor.
Xancodal may cause dependence. If taken for a long time, tolerance to the medicine may develop, requiring increasingly higher doses to maintain pain control.
Long-term use of Xancodal may lead to physical dependence, and sudden discontinuation of treatment may cause withdrawal symptoms (see section 3 "Discontinuing Xancodal"). Withdrawal symptoms may include yawning, pupil dilation, unusual or excessive tearing, runny nose, tremors, excessive sweating, anxiety, agitation, seizures, insomnia, and muscle pain.
Taking oxycodone, especially in high doses, may cause increased sensitivity to pain (hyperalgesia), which does not respond to further dose increases. It may be necessary to reduce the oxycodone dose or switch to another opioid pain reliever.
Repeated use of Xancodal may lead to dependence and abuse, which can result in life-threatening overdose. If there is a concern that the patient may become dependent on Xancodal, they should consult their doctor.
Xancodal should be used with particular caution in patients who have abused or are currently abusing alcohol or drugs.
In the event of unauthorized injection (into a vein), the medicine's excipients may cause tissue destruction (necrosis) at the injection site, changes in lung tissue (pulmonary granulomas), or other severe, potentially life-threatening events.
Breathing difficulties during sleep
Xancodal may cause breathing difficulties during sleep, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood).
Symptoms may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty staying asleep, or excessive daytime sleepiness. If the patient or another person observes such symptoms, they should contact their doctor. The doctor may consider reducing the dose.
Athletes should be warned that the medicine may give a positive result in doping tests. Using Xancodal as a doping agent can be hazardous to health.
Children
Oxycodone has not been studied in children under 12 years of age. Xancodal is not recommended for use in children under 12 years of age, as its safety and efficacy have not been established in this age group.
Xancodal and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking or has recently taken, including those they plan to take.
Certain other medicines that affect brain function (see below) may increase the risk of respiratory depression (especially in the event of overdose and in elderly patients) and/or enhance the sedative effect of Xancodal (the patient may feel extremely drowsy).
Concomitant use of Xancodal and medicines that affect brain function (e.g. sedatives such as benzodiazepines or derivatives, see below) increases the risk of drowsiness, breathing difficulties (respiratory depression), or coma, which can be life-threatening. Therefore, combination therapy should only be considered when other treatment options are not available.
If Xancodal is used with sedatives, the doctor should limit the dose and duration of concomitant use. The patient should tell their doctor about all sedatives they are taking and strictly follow the dose prescribed by their doctor.
It may be helpful to inform a relative or close friend about the possibility of the above symptoms. If such symptoms occur, the patient should consult their doctor.
Medicines that affect brain function include:
- other strong pain relievers (opioids),
- sleeping pills and sedatives (such as benzodiazepines),
- medicines used to treat depression, such as paroxetine,
- medicines used to treat allergies, motion sickness, or nausea (antihistamines or antiemetics),
- medicines used to treat mental disorders or mental illnesses (antipsychotics),
- medicines used to treat Parkinson's disease.
The risk of side effects increases if the patient is taking antidepressants (such as citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine). These medicines may interact with oxycodone, causing the patient to experience symptoms such as involuntary, rhythmic muscle contractions (including muscles that control eye movements), agitation, excessive sweating, tremors, increased reflexes, increased muscle tone, elevated body temperature above 38°C. If the patient experiences any of these symptoms, they should contact their doctor.
Additionally, interactions are possible with the following medicines:
- certain medicines that prevent blood clotting or help thin the blood (known as anticoagulants, e.g. warfarin or phenprocoumon). Xancodal may affect their action.
- muscle relaxants
- certain antibiotics (e.g. clarithromycin, erythromycin, telithromycin, or rifampicin),
- certain medicines used to treat fungal infections (e.g. ketoconazole, voriconazole, itraconazole, or posaconazole),
- certain medicines used to treat HIV infection (e.g. boceprevir, ritonavir, indinavir, nelfinavir, or saquinavir),
- cimetidine (a medicine used to treat heartburn),
- carbamazepine (a medicine used to treat epilepsy or seizures and certain pain conditions),
- phenytoin (a medicine used to treat seizures),
- St. John's Wort (a medicine used to treat depression),
- quinidine (a medicine used to treat rapid heart rate),
- monoamine oxidase inhibitors (MAOIs) taken currently or within the last two weeks (see "Warnings and precautions").
Xancodal with alcohol and drinking
Alcohol consumed while taking Xancodal may cause increased drowsiness or increase the risk of severe side effects, such as shallow breathing with a risk of respiratory arrest and loss of consciousness. It is recommended not to drink alcohol while taking Xancodal.
Drinking grapefruit juice while taking Xancodal may increase the risk of side effects. It is recommended that patients do not drink grapefruit juice while taking Xancodal.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine.
- PregnancyXancodal should not be used during pregnancy. There are limited data on the use of oxycodone in pregnant women. Oxycodone crosses the placental barrier into the fetus's blood. Long-term use of oxycodone during pregnancy may cause withdrawal symptoms in the newborn, and use during delivery may cause breathing difficulties (respiratory depression) in the newborn.
- BreastfeedingXancodal should not be used during breastfeeding, as its active substance (oxycodone) may pass into breast milk and cause drowsiness (sedation) or breathing difficulties (respiratory depression) in the breastfed child.
Driving and operating machinery
Oxycodone may impair the ability to drive or operate machinery.
General restrictions on driving may not apply to treatment with a stable dose. The treating doctor will assess each patient's situation individually.
The patient should discuss with their doctor whether and under what conditions they can drive a vehicle.
Xancodal contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per capsule, which means it is considered "sodium-free".
3. How to take Xancodal
This medicine should always be taken exactly as prescribed by the doctor. If the patient has any doubts, they should ask their doctor or pharmacist.
Recommended dose is
Adults and adolescents (from 12 years of age)
The recommended initial dose of oxycodone hydrochloride is 5 mg every 6 hours.
However, the doctor will determine the dose of the medicine needed to control the pain and how often it should be taken.
If the patient still experiences pain despite taking the medicine, they should tell their doctor.
Patients with kidney and/or liver function disorders
The usual initial dose is half the dose recommended for adults. The doctor will prescribe the most suitable dose, adapted to the patient's clinical condition, in the most suitable pharmaceutical form, if possible.
Method of administration
The medicine is intended for oral use only.
The capsules should be swallowed whole, with a large amount of liquid.
The medicine can be taken with or without food.
While taking Xancodal, the patient should not drink alcoholic beverages.
Instructions for handling a child-resistant single-dose blister pack
- 1. Do not push the capsule through the blister.
- 2. Tear off the blister strip with one capsule along the perforation.
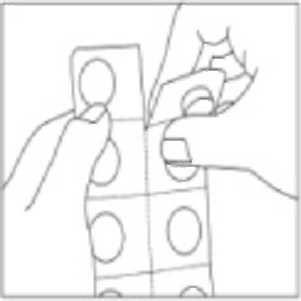
- 3. Carefully separate the foil from the back of the blister to expose the capsule.
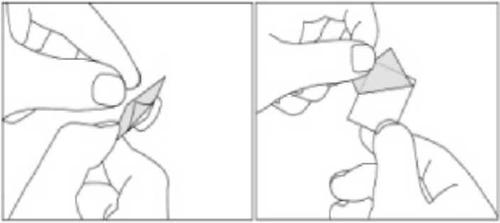
Taking a higher dose of Xancodal than recommended
If the patient takes a higher dose of Xancodal than recommended, they should immediately inform their doctor or local poison control center.
Symptoms of overdose may include:
- pupil constriction
- slow or weak breathing (respiratory depression)
- drowsiness progressing to loss of consciousness
- decreased muscle tone
- slow heart rate
- sudden drop in blood pressure
In severe cases, loss of consciousness (coma), fluid accumulation in the lungs, and circulatory collapse may occur, which can be fatal.
Under no circumstances should the patient perform tasks that require high concentration, such as driving a vehicle.
Missing a dose of Xancodal
If the patient takes a lower dose of Xancodal than recommended or misses doses, the pain-relieving effect will be insufficient or non-existent.
If the patient forgets to take a dose, they should follow the instructions below:
- If there are more than 4 hours until the next scheduled dose: take the missed dose immediately, and take subsequent doses as recommended.
- If there are less than 4 hours until the next scheduled dose: take the missed dose, and take the next dose after 4 hours. Take subsequent doses according to the recommended dosing schedule.
Do not take more than one dose in 4 hours.
Do not take a double dose to make up for a missed dose.
Discontinuing Xancodal
Do not stop treatment without consulting the doctor first.
If Xancodal is no longer necessary, it is recommended to gradually reduce the dose to prevent withdrawal symptoms.
If the patient has any further doubts about taking this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Xancodal can cause side effects, although not everybody gets them.
The most commonly reported side effects are nausea (especially at the start of treatment) and constipation. Constipation can be prevented by drinking plenty of fluids or eating foods high in fiber. If the patient experiences nausea or vomiting, the doctor may prescribe appropriate medicines.
Important side effects or symptoms to watch for and actions to take if they occur:
If the patient experiences any of the following symptoms, they should stop taking Xancodal and contact their doctor or go to the nearest emergency department immediately:
- sudden wheezing, breathing difficulties, swelling of the eyelids, face, or lips, rash, or itching (especially if it affects the whole body). These may be symptoms of severe allergic reactions.
- Slow or shallow breathing (respiratory depression). This is the most serious side effect associated with overdose of strong pain relievers like oxycodone. It occurs most often in elderly or frail patients.
- significant sudden drop in blood pressure, which may cause symptoms such as dizziness and fainting.
Possible side effects
Very common(may affect more than 1 in 10 people)
drowsiness, dizziness, headache
constipation, nausea, vomiting
itching of the skin
Common(may affect up to 1 in 10 people)
anxiety, depression, decreased activity, restlessness (mainly motor), increased activity, nervousness, sleep disturbances, abnormal thinking, confusion, tremors
lack of energy, feeling of weakness, fatigue
shortness of breath, wheezing
dry mouth, hiccups, indigestion, abdominal pain, diarrhea
decreased appetite to loss of appetite
skin rash, increased sweating
painful urination, increased need to urinate
Uncommon(may affect up to 1 in 100 people)
slow and weak breathing (respiratory depression)
allergic reactions
dehydration
agitation, mood changes, feeling of happiness
hallucinations, altered perception of reality
vision disturbances, pupil constriction
hearing impairment, feeling of dizziness or spinning
changes in taste
increased muscle tone, involuntary muscle contractions, seizures, convulsions
tingling or numbness, decreased sensation of pain or touch
coordination or balance disturbances
memory loss, concentration disturbances, speech disturbances
fainting
rapid heart rate, palpitations (in connection with withdrawal syndrome)
vasodilation causing a drop in blood pressure
coughing, voice changes
mouth ulcers, gum soreness
gas, difficulty swallowing, reflux
intestinal obstruction
decreased sex drive, impotence, low sex hormone levels in the blood detected in laboratory tests (hypogonadism)
accidents
general feeling of being unwell, pain (e.g. in the chest)
swelling of hands, ankles, or feet
migraine
tolerance to the medicine
dry skin
thirst
difficulty urinating
chills
physical dependence, including withdrawal symptoms (see section 3 "Discontinuing Xancodal")
increased liver enzyme activity (detected in laboratory tests)
Rare(may affect up to 1 in 1000 people)
low blood pressure, dizziness, fainting due to sudden drop in blood pressure when standing up
gum bleeding, increased appetite, black stools, changes in teeth
blistering of the skin and mucous membranes (urticaria), hives
weight changes (decrease or increase)
Frequency not known(cannot be estimated from the available data)
absence of menstruation
severe allergic reaction causing breathing difficulties or dizziness
aggression
increased sensitivity to pain (hyperalgesia)
tooth decay
gallstones (causing abdominal pain), bile stasis
withdrawal symptoms in newborns
dependence on the medicine
smooth muscle spasms
suppressed cough reflex
- sleep apnea (pauses in breathing during sleep)
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw
tel.: +48 22 49 21 301, fax: +48 22 49 21 309, e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Xancodal
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after "EXP". The expiry date refers to the last day of the month.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer use. This will help protect the environment.
6. Package contents and other information
What Xancodal contains
The active substance is oxycodone hydrochloride.
Xancodal, 5 mg
Each hard capsule contains 5 mg of oxycodone hydrochloride, equivalent to 4.48 mg of oxycodone.
Xancodal, 10 mg
Each hard capsule contains 10 mg of oxycodone hydrochloride, equivalent to 8.96 mg of oxycodone.
Xancodal, 20 mg
Each hard capsule contains 20 mg of oxycodone hydrochloride, equivalent to 17.93 mg of oxycodone.
Other ingredients are:
Capsule contents: microcrystalline cellulose, magnesium stearate
Capsule shell: gelatin, sodium lauryl sulfate, titanium dioxide (E171), yellow iron oxide (E172), red iron oxide (E172), indigo carmine (E132)
Printing ink: shellac, black iron oxide (E172), potassium hydroxide (to adjust pH).
What Xancodal looks like and contents of the pack
Xancodal, 5 mg
Hard capsules, 14.4 mm in length, with a dark pink body and "5" printed on it and a brown cap with "OXY" printed on it.
Xancodal, 10 mg
Hard capsules, 14.4 mm in length, with a white body and "10" printed on it and a brown cap with "OXY" printed on it.
Xancodal, 20 mg
Hard capsules, 14.4 mm in length, with a light pink body and "20" printed on it and a brown cap with "OXY" printed on it.
Pack sizes:
Child-resistant single-dose blisters in a cardboard box containing 20x1, 30x1, 50x1, and 100x1 hard capsules.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
Manufacturer
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben, Germany
Activis ehf.
Reykjavikurvegur 78
220 Hafnarfjordur, Iceland
Balkanpharma Dupnitsa AD
3 Samokovsko Shosse Str.
2600 Dupnitsa, Bulgaria
Lek Pharmaceuticals d.d.,
Verovškova ulica 57,
1526 Ljubljana, Slovenia
For more information about this medicine and its names in other European Economic Area countries, please contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
tel. +48 22 209 70 00
Date of last revision of the leaflet:05/2022
Sandoz logo
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterActavis ehf. Balkanpharma - Dupnitsa AD Lek Pharmaceuticals d.d. Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Xancodal
Alternatives to Xancodal in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Xancodal in Ukraine
Alternative to Xancodal in Spain
Online doctors for Xancodal
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Xancodal – subject to medical assessment and local rules.














