
Vitaminum B12 Vzf

Ask a doctor about a prescription for Vitaminum B12 Vzf

How to use Vitaminum B12 Vzf
Leaflet attached to the packaging: patient information
VITAMINUM B WZF 100 micrograms/ml, solution for injection
VITAMINUM B WZF 500 micrograms/ml, solution for injection
Cyanocobalamin
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Vitaminum B WZF and what is it used for
- 2. Important information before using Vitaminum B WZF
- 3. How to use Vitaminum B WZF
- 4. Possible side effects
- 5. How to store Vitaminum B WZF
- 6. Contents of the packaging and other information
1. What is Vitaminum B WZF and what is it used for
Vitaminum B WZF contains cyanocobalamin (vitamin B12), which is essential for the growth process of the body. It affects the formation of blood cells and the proper functioning of the nervous system. A deficiency of vitamin B12 may cause the development of megaloblastic anemia and irreversible neurological complications.
The medicine is used:
- in megaloblastic anemia of Addison-Biermer (a specific type of anemia resulting from a lack of vitamin B12);
- in other megaloblastic anemias with a vitamin B12 deficiency;
- in vitamin B12 deficiencies due to:
- -complete exclusion of animal products from the diet (strict vegetarianism);
- -congenital or acquired impairment of the production of intrinsic factor enabling the absorption of vitamin B12 (Castle's factor);
- -lack of Castle's factor due to gastric resection;
- -chronic atrophic gastritis;
- -malabsorption syndromes after resection of the ileum, in the course of celiac disease (a disease caused by gluten intolerance), tropical sprue (a disease of the digestive tract leading to disturbances in the absorption of nutrients), Crohn's disease (an inflammatory disease of the digestive tract);
- -utilization of vitamin B12 by intestinal bacteria (blind loop syndrome) or parasites (infection with Diphyllobothrium latum);
- in the Schilling test (a test of vitamin B12 absorption).
2. Important information before using Vitaminum B WZF
When not to use Vitaminum B WZF
- if the patient is allergic to vitamin B12, cobalt, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Vitaminum B WZF, the patient should discuss it with their doctor or pharmacist.
- In patients with Leber's disease (visual disturbances due to optic nerve atrophy), the medicine should be used with caution, as cyanocobalamin may accelerate optic nerve atrophy.
- In patients with megaloblastic anemia (during treatment with high doses of vitamin B12), potassium levels in the blood may decrease.
- In Addison-Biermer's disease, vitamin B12 should be used regularly throughout life. A few months' break in administration, seemingly justified by good blood test results (morphology), may lead to irreversible damage to the nervous system.
- Treatment with vitamin B12 may reveal symptoms of true erythema (severe redness of the face, hands, feet, and mucous membranes).
- Before starting treatment and between the 5th and 7th day of treatment, it is recommended to perform the following laboratory tests: folic acid levels, hematocrit, reticulocytes (a significant increase in the number of reticulocytes - young red blood cells is an early sign of treatment efficacy), and vitamin B12 levels. In the case of long-term treatment, it is recommended to monitor hematological parameters (blood condition) and vitamin B12 levels every 3 to 6 months.
Vitaminum B WZF and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
- Certain antibiotics, methotrexate (used in cancer treatment), or pyrimethamine (used in malaria treatment) may affect vitamin B12 levels in serum, giving false-negative results.
- Colchicine (used in gout treatment), para-aminosalicylic acid (an anti-tuberculosis medicine), and consumption of large amounts of alcohol for more than 2 weeks may lead to disturbances in vitamin B12 absorption.
- Concomitant use of medicines that suppress bone marrow activity (e.g., chloramphenicol) may weaken the response to vitamin B12 treatment.
- Folic acid used in high doses for a long time may decrease vitamin B12 levels in the blood.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine may be used during pregnancy only in cases where the doctor believes the benefits to the mother outweigh the potential risks to the fetus.
No adverse effects have been observed in children breastfed by women taking the recommended doses of vitamin B12.
Driving and using machines
The medicine does not affect the ability to drive or use machines.
Vitaminum B WZF contains sodium
Vitaminum B WZF 100 micrograms/ml
The medicine contains 3.96 mg of sodium (the main component of common salt) per 1 ml ampoule. This corresponds to 0.2% of the maximum recommended daily intake of sodium in the diet for adults.
Vitaminum B WZF 500 micrograms/ml
The medicine contains less than 1 mmol (23 mg) of sodium per 2 ml ampoule, which means the medicine is considered "sodium-free".
3. How to use Vitaminum B WZF
This medicine should always be used according to the doctor's recommendations. In case of doubts, consult a doctor or pharmacist.
The medicine should be administered by intramuscular or deep subcutaneous injection.
The medicine must not be administered intravenously. The dosage and method of administration are determined by the doctor.
Detailed dosing and instructions for use and preparation of the medicine for administration are provided at the end of the leaflet, in the section "Information intended exclusively for healthcare professionals".
Using a higher dose of Vitaminum B WZF than recommended
If the patient suspects they have received too high a dose of the medicine, they should inform their doctor.
There are no known cases of overdose of the medicine.
4. Possible side effects
Like all medicines, Vitaminum B WZF can cause side effects, although not everybody gets them.
The following side effects may occur during treatment with the medicine:
- cases of anaphylactic shock (symptoms: circulatory disorders, blood pressure drop, facial, lip, tongue, and throat swelling, causing difficulty breathing or swallowing, hives) and deaths after parenteral administration of vitamin B12, hypersensitivity (very rare);
- pulmonary edema, congestive heart failure (symptoms: body swelling, fatigue, fluid accumulation in body cavities);
- peripheral vascular thrombosis;
- true erythema;
- itching, rash;
- feeling of bloating, mild transient diarrhea;
- pain after intramuscular injection.
Reporting side effects
If any side effects occur, including any not listed in this leaflet, the patient should inform their doctor or pharmacist.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Tel.: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Vitaminum B WZF
Store the ampoules in the outer packaging to protect them from light, at a temperature below 25°C. Do not freeze.
Store the medicine out of the sight and reach of children.
Do not use the medicine after the expiry date stated on the carton and ampoule.
The expiry date stated on the packaging means the last day of the stated month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Vitaminum B WZF contains
- The active substance of the medicine is cyanocobalamin. Each ml of the solution contains 100 or 500 micrograms of cyanocobalamin (vitamin B12).
- The other ingredients are: sodium acetate trihydrate, glacial acetic acid, sodium chloride, water for injections.
What Vitaminum B WZF looks like and what the packaging contains
Vitaminum B WZF is a pink, clear liquid.
Packaging:
Vitaminum B WZF 100 micrograms/ml: 10 ampoules of 1 ml each in a cardboard box.
Vitaminum B WZF 500 micrograms/ml: 5 ampoules of 2 ml each in a cardboard box.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
tel. +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
Date of last revision of the leaflet: ---------------------------------------------------------------------------
Information intended exclusively for healthcare professionals:
VITAMINUM B WZF 100 micrograms/ml, solution for injection
VITAMINUM B WZF 500 micrograms/ml, solution for injection
Cyanocobalamin
The medicine should be administered by intramuscular or deep subcutaneous injection.
The medicine must not be administered intravenously.
It is not recommended to mix the vitamin B12 solution with sodium warfarin solution for injection.
Vitamin C destroys cyanocobalamin in vitro, so it is recommended to avoid simultaneous administration of these vitamins.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
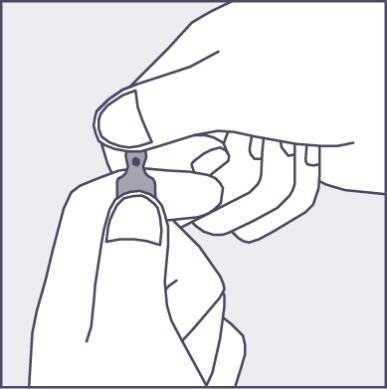
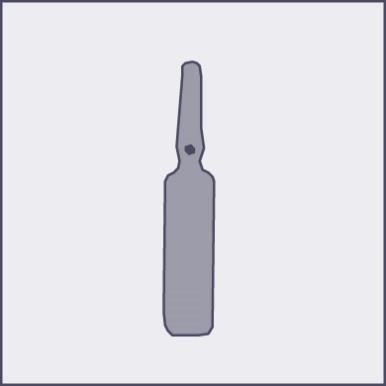
A colored dot is placed on each ampoule (see figure 1) as a mark indicating the location of the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
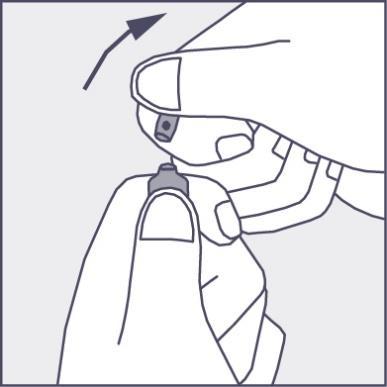
- Press in the direction of the arrow shown in figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused product should be destroyed in accordance with applicable regulations.
Figure 1
Figure 2
Figure 3
Dosage
Adults:
Addison-Biermer's anemia without neurological symptoms:
250 to 1000 micrograms every other day for 1 to 2 weeks, then 250 micrograms once a week, until hematological improvement is achieved. Maintenance dose: 1000 micrograms once a month.
In the case of neurological complications (so-called funicular myelosis): 1000 micrograms every other day, until improvement is achieved.
Vitamin B12 deficiencies after gastric resection or due to malabsorption: 250 to 1000 micrograms once a month.
Children:
Addison-Biermer's anemia without neurological symptoms:
up to 3 years:
5 to 15 micrograms per day;
3-18 years:
15 to 30 micrograms per day.
Megaloblastic anemia:
up to 3 years:
5 to 15 micrograms per week;
3-7 years:
15 to 25 micrograms per week;
7-18 years:
15 to 30 micrograms per week.
Anemia with neurological symptoms:
up to 3 years:
100 to 200 micrograms per week;
3-7 years:
200 to 500 micrograms per week;
7-18 years:
500 to 1000 micrograms per week.
Schilling test
After oral administration of labeled vitamin B12, 1000 micrograms of vitamin B12 are administered intramuscularly as a single dose.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Vitaminum B12 VzfDosage form: Tablets, 1000 mcgActive substance: cyanocobalaminPrescription not requiredDosage form: Tablets, 1000 mcgActive substance: cyanocobalaminPrescription requiredDosage form: Solution, 100 mcg/mlActive substance: cyanocobalaminPrescription required
Alternatives to Vitaminum B12 Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vitaminum B12 Vzf in Spain
Alternative to Vitaminum B12 Vzf in Ukraine
Online doctors for Vitaminum B12 Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vitaminum B12 Vzf – subject to medical assessment and local rules.














