
Urapidil Kalceks

Ask a doctor about a prescription for Urapidil Kalceks

How to use Urapidil Kalceks
Package Leaflet: Information for the User
Urapidil KALCEKS, 25 mg, Solution for Injection/Infusion
Urapidil KALCEKS, 50 mg, Solution for Injection/Infusion
Urapidil
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- This leaflet should be kept in case it needs to be read again.
- In case of any further doubts, consult a doctor or nurse.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What is Urapidil KALCEKS and what is it used for
- 2. Important information before using Urapidil KALCEKS
- 3. How to use Urapidil KALCEKS
- 4. Possible side effects
- 5. How to store Urapidil KALCEKS
- 6. Contents of the pack and other information
1. What is Urapidil KALCEKS and what is it used for
Urapidil KALCEKS contains the active substance urapidil. Urapidil is a blood pressure lowering medicine (antihypertensive) belonging to a group of medicines called "alpha-adrenergic receptor blockers". This medicine works by affecting blood vessels (i.e., arteries and veins). It lowers blood pressure by reducing the tension of blood vessels.
This medicine is used in adults:
- in situations of sudden high blood pressure (e.g., sudden, severe high blood pressure called "hypertensive crisis");
- to treat severe to very severe high blood pressure or high blood pressure that does not respond to treatment;
- to lower high blood pressure during and after surgical procedures.
2. Important information before using Urapidil KALCEKS
When not to use Urapidil KALCEKS
- if the patient is allergic to urapidil or any of the other ingredients of this medicine (listed in section 6);
- if the patient has narrowing of the main artery (aortic stenosis) or vascular abnormalities called "vascular shunt" (except for arteriovenous shunt in dialysis patients);
- if the patient is breastfeeding.
Warnings and precautions
If blood pressure drops too quickly, it may cause a slow heart rate or cardiac arrest.
Before taking this medicine, the patient should discuss it with their doctor or nurse if any of the following points apply to them, as special caution is recommended:
- if the patient has had diarrhea or vomiting (or other conditions that cause a decrease in body fluids);
- in patients with heart failure caused by mechanical damage, e.g., narrowing of the heart valve (aortic or mitral stenosis);
- in patients with pulmonary artery obstruction (pulmonary embolism);
- in patients with heart rhythm disorders caused by pericarditis (pericardial disease);
- in patients with liver disorders;
- in patients with moderate to severe kidney disorders;
- in elderly patients;
- in patients taking cimetidine (a medicine that inhibits stomach acid production) at the same time. If the patient is unsure whether any of the above applies to them, they should consult their doctor or nurse.
If the patient is going to have eye surgery for cataracts (clouding of the lens), they should inform their ophthalmologist before the surgery that they are taking or have taken urapidil. This is because urapidil may cause complications during surgery that can be prevented if the specialist is prepared in advance.
If another blood pressure lowering medicine has been given before urapidil, the doctor will wait long enough for the previous medicine to take effect. The doctor will reduce the dose of urapidil. Too rapid a drop in blood pressure may lead to a slow heart rate or cardiac arrest.
Children
This medicine should not be used in children and adolescents.
Urapidil KALCEKS and other medicines
The patient should tell their doctor or nurse about all medicines they are taking or have recently taken, as well as any medicines they plan to take.
Before taking this medicine, the patient should inform their doctor or nurse if they are taking any of the following medicines, as they may interact with Urapidil KALCEKS, changing the effect of the medicines or increasing the risk of side effects:
- alpha-adrenergic receptor blockers (used to treat urinary disorders related to prostate disease);
- any blood pressure lowering medicines;
- cimetidine (used to inhibit stomach acid production);
- barbiturates (medicines used to treat epilepsy).
Urapidil KALCEKS with alcohol
Alcohol may enhance the effect of this medicine.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before taking this medicine.
There is not enough data to assess the safety of urapidil in pregnant women.
This medicine should not be used during pregnancy unless it is necessary due to the woman's clinical condition. If high blood pressure occurs during pregnancy and treatment with this medicine is necessary, blood pressure should be lowered gradually and always under medical supervision.
It is not known whether the medicine passes into breast milk. For safety reasons, this medicine should not be used during breastfeeding.
This medicine is not recommended for women of childbearing age who do not use contraception.
Animal studies have shown that urapidil affects fertility. However, the significance of this effect in humans is not known.
Driving and using machines
This medicine may affect the ability to drive and use machines, especially when starting treatment, increasing the dose, changing treatment, or in combination with alcohol.
Urapidil KALCEKS contains propylene glycol and sodium
Propylene glycol:
- This medicine contains 500 mg of propylene glycol per 5 ml of solution and 1,000 mg of propylene glycol per 10 ml of solution, which corresponds to 100 mg/ml.
- If the patient is pregnant or breastfeeding, this medicine should not be given unless prescribed by a doctor. The doctor may perform additional checks during treatment with this medicine.
- If the patient has liver or kidney disease, they should not take this medicine unless prescribed by a doctor. The doctor may perform additional checks during treatment with this medicine.
- Propylene glycol in this medicine may cause symptoms similar to those after alcohol consumption and increase the risk of side effects. Sodium:
- The medicine contains less than 1 mmol (23 mg) of sodium per ml, which means the medicine is considered "sodium-free".
3. How to use Urapidil KALCEKS
How to use the medicine
- This medicine will be given by medical professionals.
- This medicine will be given by injection or intravenous infusion. It can be given as a single or multiple injections or as a continuous infusion. Injections can be combined (continued) with continuous infusions.
- During administration of this medicine, the patient should be in a lying position.
- During treatment, the patient's blood pressure will be constantly monitored.
Dosage
The doctor will decide on the appropriate dose based on the patient's condition.
Hypertensive crisis and severe to very severe high blood pressure or resistant high blood pressure
Intravenous injection
10-50 mg of urapidil is given slowly - with constant blood pressure monitoring. A decrease in blood pressure can be expected within 5 minutes after injection. Depending on the reaction of blood pressure, the injection of urapidil can be repeated.
Intravenous infusion (as a drip or using an infusion pump)
In the case of continuous intravenous infusion, 250 mg of urapidil is added to 500 ml of compatible infusion solution (0.9% sodium chloride or 5% or 10% glucose solution).
In the case of using an infusion pump, 100 mg of urapidil is drawn into the infusion pump and diluted to a volume of 50 ml with a compatible infusion solution (see above) (maximum 4 mg of urapidil per ml of infusion solution).
The initial infusion rate is 2 mg/min. The maintenance dose is approximately 9 mg/hour.
The degree of blood pressure reduction will be determined based on the dose given in the first 15 minutes. The desired blood pressure level can then be maintained at significantly lower doses.
Reducing high blood pressure during and after surgery
To maintain the blood pressure level achieved by injection, a continuous infusion is used, either using an infusion pump or continuous intravenous infusion.
By intravenous injection
Initially, 25 mg of urapidil is given. This dose will be repeated if, after 2 minutes, there is no sufficient decrease in blood pressure. If, within 2 minutes after the second dose, the decrease in blood pressure is still insufficient, 50 mg of urapidil will be given.
If the decrease in blood pressure after 2 minutes from the administration of the dose is sufficient, the patient will receive a maintenance dose.
Intravenous infusion (as a drip or using an infusion pump)
Initially, up to 6 mg will be given over 1-2 minutes, then the dose will be reduced.
Special patient groups
In patients with liver and/or kidney disease, it may be necessary to reduce the dose.
In elderly patients, this medicine must be given with special caution, initially in small doses, due to the changed sensitivity of these patients to medicines of this group.
Duration of treatment
The duration of treatment with this medicine should not exceed 7 days.
Overdose of Urapidil KALCEKS
In case of administration of too high a dose of this medicine, the patient may experience dizziness, a feeling of emptiness in the head, or fainting when standing up, fatigue, and slowed reaction time. In such a case, the patient should lie down on their back with their legs raised. If the symptoms do not disappear, the doctor or nurse should be informed immediately.
In case of any doubts about the use of this medicine, the doctor or nurse should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most of the side effects listed below were related to too rapid a decrease in blood pressure; however, experience to date has shown that even during slow infusion, they usually disappear within a few minutes. The doctor will decide whether to stop or not stop treatment, depending on the severity of the side effects.
Common(may affect up to 1 in 10 people)
Dizziness, headache, nausea.
Uncommon(may affect up to 1 in 100 people)
Sleep disorders, palpitations, decreased or increased heart rate, feeling of pressure or pain in the chest (as in angina pectoris), breathing difficulties, drop in blood pressure when standing up from a sitting or lying position (orthostatic hypotension), vomiting, diarrhea, dry mouth, sweating, fatigue, irregular heartbeat.
Rare(may affect up to 1 in 1,000 people)
Nasal congestion, allergic reactions (itching, redness of the skin, rash), prolonged and painful erection.
Very rare(may affect up to 1 in 10,000 people)
Anxiety, increased need to urinate, severe incontinence, decreased platelet count (blood cells involved in blood clotting).
Frequency not known(frequency cannot be estimated from the available data)
Hives, severe allergic reaction with swelling of the face, lips, tongue, and throat.
Reporting side effects
If side effects occur, the doctor or nurse should be informed.
This includes any side effects not listed in the leaflet.
Side effects can also be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Urapidil KALCEKS
The medicine should be stored out of sight and reach of children.
There are no special precautions for storage of the medicinal product.
After dilution
Chemical and physical stability has been demonstrated for 50 hours at 25°C and 2-8°C after dilution in 9 mg/ml (0.9%) sodium chloride or 50 mg/ml (5%) or 100 mg/ml (10%) glucose solution for infusion.
From a microbiological point of view, the diluted solution should be used immediately. If not used immediately, the user is responsible for the storage time and conditions before use, which usually should not exceed 24 hours at 2-8°C, unless the dilution was performed under controlled and validated aseptic conditions..
Do not use this medicine after the expiry date stated on the carton after "EXP" and on the ampoule after "EXP". The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Urapidil KALCEKS contains
- The active substance is urapidil. 1 ml of solution contains 5 mg of urapidil. Each 5 ml ampoule contains 25 mg of urapidil. Each 10 ml ampoule contains 50 mg of urapidil.
- The other ingredients are: hydrochloric acid, sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, propylene glycol, sodium hydroxide (for pH adjustment), water for injections.
What Urapidil Kalceks looks like and contents of the pack
Ampoules of colorless glass type I, with a capacity of 5 ml or 10 ml with a break point (one point cut). 5 ampoules are packed in a blister pack. The whole is packed in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
AS KALCEKS
Krustpils iela 71E
LV-1057 Rīga
Latvia
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Estonia, Czech Republic, Italy, Portugal
Urapidil Kalceks
Austria
Urapidil Kalceks 25 mg, 50 mg Injektions-/Infusionslösung
Germany
Urapidil Ethypharm 25 mg, 50 mg Injektions-/Infusionslösung
Spain
Urapidil Kalceks 5 mg/ml solución inyectable y para perfusion
France
URAPIDIL KALCEKS 25 mg/5 ml, solution injectable/pour perfusion
URAPIDIL KALCEKS 50 mg/10 ml, solution injectable/pour perfusion
Hungary
Urapidil Kalceks 25 mg, 50 mg oldatos injekció vagy infúzió
Latvia
Urapidil Kalceks 25 mg, 50 mg šķīdums injekcijām/infūzijām
Netherlands
Urapidil Kalceks 25 mg, 50 mg oplossing voor injectie/infusie
Poland
Urapidil KALCEKS
Romania
Urapidil Kalceks 25 mg, 50 mg soluție injectabilă/perfuzabilă
Slovakia
Urapidil Kalceks 25 mg, 50 mg injekčný/infúzny roztok
Date of last revision of the leaflet: 01/2022
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Dosage
Acute conditions in hypertension (e.g., hypertensive crisis), severe to very severe hypertension or resistant hypertension
- Intravenous injection10-50 mg of urapidil is given slowly - with constant blood pressure monitoring. A decrease in blood pressure can be expected within 5 minutes after injection. Depending on the reaction of blood pressure, the injection of urapidil can be repeated.
- Intravenous infusion or continuous infusion using an infusion pump
Intravenous infusion (as a drip or using an infusion pump) is used to maintain blood pressure achieved by injecting the product. To obtain instructions for preparing the diluted solution, see "Instructions for use and disposal" and "Preparation of the diluted solution" below.
The maximum amount of urapidil is 4 mg per ml of infusion solution.
Rate of administration
The infusion rate depends on the individual blood pressure values of the patient.
The initial infusion rate is: 2 mg/min.
The degree of blood pressure reduction depends on the dose given in the first 15 minutes. The desired blood pressure level can then be maintained at significantly lower doses.
Maintenance dose: approximately 9 mg/hour, which corresponds to 250 mg of urapidil added to 500 ml of infusion solution, which corresponds to 1 mg = 44 drops = 2.2 ml.
Controlled reduction of blood pressure in case of increased blood pressure during and (or) after surgery
To maintain the blood pressure level achieved by injection, a continuous infusion is used, either using an infusion pump or continuous intravenous infusion.
Dosing schedule
Intravenous injection
25 mg of urapidil
(= 5 ml of injection/infusion solution)
If blood pressure reduction occurs
after 2 minutes.
Blood pressure at
stabilized level through
infusion
Initially, up to
6 mg will be given over
1-2 minutes, then the
dose will be reduced
no change in blood
pressure after 2 minutes.
Intravenous injection
25 mg of urapidil
(= 5 ml of injection/infusion solution)
If blood pressure reduction occurs
after 2 minutes.
after 2 minutes.
no change in blood
pressure after 2 minutes.
If blood pressure reduction occurs
after 2 minutes.
Slow intravenous injection
50 mg of urapidil
(= 10 ml of injection solution)
Special patient groups
In patients with liver and/or kidney disease, it may be necessary to reduce the dose of urapidil.
In elderly patients, antihypertensive medicines must be given with special caution, initially in small doses, due to the changed sensitivity of these patients to medicines of this group.
Method of administration
Intravenous administration.
Urapidil KALCEKS is given intravenously by injection or infusion, and the patient should be in a lying position. The dose is given as a single or multiple injections, as well as a slow infusion. Injections can be combined (continued) with slow infusions.
When switching to oral therapy, it is possible to change to maintenance therapy with oral antihypertensive medicines.
To ensure safety against toxicological effects, treatment should not be used for more than 7 days, which also applies to parenteral antihypertensive therapy.
In case of recurrence of hypertension, parenteral treatment can be repeated.
Incompatibilities
This medicinal product must not be mixed with alkaline injection or infusion solutions, as they may become cloudy or precipitate due to the acidic properties of the solution.
This medicinal product must not be mixed with other medicinal products except those listed below.
Instructions for use and disposal
For single use only.
Use immediately after opening the ampoule. All unused remnants of the medicinal product or waste should be disposed of.
This medicinal product should be visually inspected before use. Only a clear and particle-free solution can be administered.
Preparation of the diluted solution
- Intravenous injection:250 mg of urapidil should be added to 500 ml of compatible infusion solution (see below).
- Infusion pump:20 ml of injection/infusion solution (= 100 mg of urapidil) should be drawn into the infusion pump and diluted to a volume of 50 ml with a compatible infusion solution (see below).
It can be diluted with:
- 9 mg/ml (0.9%) sodium chloride solution for infusion;
- 50 mg/ml (5%) glucose solution for infusion;
- 100 mg/ml (10%) glucose solution for infusion.
Instructions for opening the ampoule
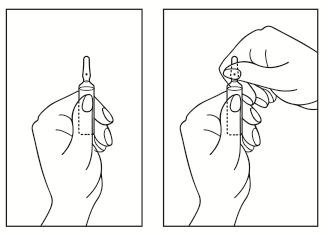
- 1) Turn the ampoule with the colored point upwards. If there is a solution in the upper part of the ampoule, gently tap with your finger to move the entire solution to the lower part of the ampoule.
- 2) Use both hands to open the ampoule; holding the lower part of the ampoule in one hand, break the upper part of the ampoule in the direction away from the colored point (see picture below).
Any unused remnants of the medicinal product or waste should be disposed of in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAS Kalceks
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Urapidil KalceksDosage form: Solution, 5 mg/mlActive substance: urapidilManufacturer: Takeda Austria GmbHPrescription not requiredDosage form: Solution, 25 mgActive substance: urapidilManufacturer: Cenexi EVER Neuro Pharma GmbHPrescription requiredDosage form: Solution, 50 mgActive substance: urapidilManufacturer: Cenexi EVER Neuro Pharma GmbHPrescription required
Alternatives to Urapidil Kalceks in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Urapidil Kalceks in Ukraine
Alternative to Urapidil Kalceks in Spain
Online doctors for Urapidil Kalceks
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Urapidil Kalceks – subject to medical assessment and local rules.










